Update on a Pharmacokinetic-Centric Alternative Tier II Program for MMT-Part II: Physiologically Based Pharmacokinetic Modeling and Manganese Risk Assessment
- PMID: 22645610
- PMCID: PMC3356703
- DOI: 10.1155/2012/791431
Update on a Pharmacokinetic-Centric Alternative Tier II Program for MMT-Part II: Physiologically Based Pharmacokinetic Modeling and Manganese Risk Assessment
Abstract
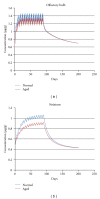
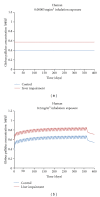
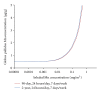
Recently, a variety of physiologically based pharmacokinetic (PBPK) models have been developed for the essential element manganese. This paper reviews the development of PBPK models (e.g., adult, pregnant, lactating, and neonatal rats, nonhuman primates, and adult, pregnant, lactating, and neonatal humans) and relevant risk assessment applications. Each PBPK model incorporates critical features including dose-dependent saturable tissue capacities and asymmetrical diffusional flux of manganese into brain and other tissues. Varied influx and efflux diffusion rate and binding constants for different brain regions account for the differential increases in regional brain manganese concentrations observed experimentally. We also present novel PBPK simulations to predict manganese tissue concentrations in fetal, neonatal, pregnant, or aged individuals, as well as individuals with liver disease or chronic manganese inhalation. The results of these simulations could help guide risk assessors in the application of uncertainty factors as they establish exposure guidelines for the general public or workers.
Figures



Similar articles
-
Update on a Pharmacokinetic-Centric Alternative Tier II Program for MMT-Part I: Program Implementation and Lessons Learned.J Toxicol. 2012;2012:946742. doi: 10.1155/2012/946742. Epub 2012 Mar 27. J Toxicol. 2012. PMID: 22545047 Free PMC article.
-
Manganese tissue dosimetry in rats and monkeys: accounting for dietary and inhaled Mn with physiologically based pharmacokinetic modeling.Toxicol Sci. 2009 Mar;108(1):22-34. doi: 10.1093/toxsci/kfn264. Epub 2008 Dec 19. Toxicol Sci. 2009. PMID: 19098275
-
Pharmacokinetic modeling of manganese in the rat IV: Assessing factors that contribute to brain accumulation during inhalation exposure.J Toxicol Environ Health A. 2008;71(7):413-26. doi: 10.1080/15287390701838697. J Toxicol Environ Health A. 2008. PMID: 18306088
-
Risk assessment of an essential element: manganese.J Toxicol Environ Health A. 2010;73(2):128-55. doi: 10.1080/15287390903337118. J Toxicol Environ Health A. 2010. PMID: 20077284 Review.
-
Use of physiologically based pharmacokinetic modeling to support development of an acute (24-hour) health-based inhalation guideline for manganese.Regul Toxicol Pharmacol. 2023 Dec;145:105518. doi: 10.1016/j.yrtph.2023.105518. Epub 2023 Oct 19. Regul Toxicol Pharmacol. 2023. PMID: 37863417 Review.
Cited by
-
Incorporation of rapid association/dissociation processes in tissues into the monkey and human physiologically based pharmacokinetic models for manganese.Toxicol Sci. 2023 Feb 17;191(2):212-226. doi: 10.1093/toxsci/kfac123. Toxicol Sci. 2023. PMID: 36453847 Free PMC article.
-
Changes in Brain Metallome/Metabolome Pattern due to a Single i.v. Injection of Manganese in Rats.PLoS One. 2015 Sep 18;10(9):e0138270. doi: 10.1371/journal.pone.0138270. eCollection 2015. PLoS One. 2015. PMID: 26383269 Free PMC article.
-
Exposure to Mixtures of Metals and Neurodevelopmental Outcomes: A Multidisciplinary Review Using an Adverse Outcome Pathway Framework.Risk Anal. 2015 Jun;35(6):971-1016. doi: 10.1111/risa.12425. Epub 2015 Jun 10. Risk Anal. 2015. PMID: 26096925 Free PMC article.
-
Mechanisms of lead and manganese neurotoxicity.Toxicol Res (Camb). 2013 Mar 1;2(2):99-114. doi: 10.1039/C2TX20064C. Toxicol Res (Camb). 2013. PMID: 25722848 Free PMC article.
-
Principles of dose-setting in toxicology studies: the importance of kinetics for ensuring human safety.Arch Toxicol. 2021 Dec;95(12):3651-3664. doi: 10.1007/s00204-021-03155-4. Epub 2021 Oct 8. Arch Toxicol. 2021. PMID: 34623454 Free PMC article. Review.
References
-
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Critical Reviews in Toxicology. 2005;35(1):1–32. - PubMed
-
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Manganese. U.S. Department of Health and Human Services, Atlanta, Ga, USA, 2008, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=102&tid=23.
-
- U.S. Environmental Protection Agency. Reevaluation of inhalation risks associated with methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline. EPA Office of Research and Development. EPA Air Docket A-93-26 No. II-A-12, 1994.
LinkOut - more resources
Full Text Sources

