Opposing role of condensin hinge against replication protein A in mitosis and interphase through promoting DNA annealing
- PMID: 22645654
- PMCID: PMC3352087
- DOI: 10.1098/rsob.110023
Opposing role of condensin hinge against replication protein A in mitosis and interphase through promoting DNA annealing
Abstract
Condensin is required for chromosome dynamics and diverse DNA metabolism. How condensin works, however, is not well understood. Condensin contains two structural maintenance of chromosomes (SMC) subunits with the terminal globular domains connected to coiled-coil that is interrupted by the central hinge. Heterotrimeric non-SMC subunits regulate SMC. We identified a novel fission yeast SMC hinge mutant, cut14-Y1, which displayed defects in DNA damage repair and chromosome segregation. It contains an amino acid substitution at a conserved hinge residue of Cut14/SMC2, resulting in diminished DNA binding and annealing. A replication protein A mutant, ssb1-418, greatly alleviated the repair and mitotic defects of cut14-Y1. Ssb1 protein formed nucleolar foci in cut14-Y1 cells, but the number of foci was diminished in cut14-Y1 ssb1-418 double mutants. Consistent with the above results, Ssb1 protein bound to single-strand DNA was removed by condensin or the SMC dimer through DNA reannealing in vitro. Similarly, RNA hybridized to DNA may be removed by the SMC dimer. Thus, condensin may wind up DNA strands to unload chromosomal components after DNA repair and prior to mitosis. We show that 16 suppressor mutations of cut14-Y1 were all mapped within the hinge domain, which surrounded the original L543 mutation site.
Keywords: structural maintenance of chromosomes, DNA damage, mitosis, DNA metabolism, condensation.
Figures



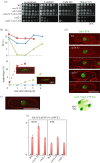



References
-
- Wood AJ, Severson AF, Meyer BJ. 2010. Condensin and cohesin complexity: the expanding repertoire of functions. Nat. Rev. Genet. 11, 391–40410.1038/nrg2794 (doi:10.1038/nrg2794) - DOI - DOI - PMC - PubMed
-
- Hudson DF, Marshall KM, Earnshaw WC. 2009. Condensin: architect of mitotic chromosomes. Chromosome Res. 17, 131–14410.1007/s10577-008-9009-7 (doi:10.1007/s10577-008-9009-7) - DOI - DOI - PubMed
-
- Anderson DE, Losada A, Erickson HP, Hirano T. 2002. Condensin and cohesin display different arm conformations with characteristic hinge angles. J. Cell Biol. 156, 419–42410.1083/jcb.200111002 (doi:10.1083/jcb.200111002) - DOI - DOI - PMC - PubMed
-
- Yoshimura SH, Hizume K, Murakami A, Sutani T, Takeyasu K, Yanagida M. 2002. Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr. Biol. 12, 508–51310.1016/S0960-9822(02)00719-4 (doi:10.1016/S0960-9822(02)00719-4) - DOI - DOI - PubMed
-
- Hirano M, Hirano T. 2002. Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J. 21, 5733–574410.1093/emboj/cdf575 (doi:10.1093/emboj/cdf575) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

