The role of non-canonical SNAREs in synaptic vesicle recycling
- PMID: 22645707
- PMCID: PMC3355972
- DOI: 10.4161/cl.20114
The role of non-canonical SNAREs in synaptic vesicle recycling
Abstract
An increasing number of studies suggest that distinct pools of synaptic vesicles drive specific forms of neurotransmission. Interspersed with these functional studies are analyses of the synaptic vesicle proteome which have consistently detected the presence of so-called "non-canonical" SNAREs that typically function in fusion and trafficking of other subcellular structures within the neuron. The recent identification of certain non-canonical vesicular SNAREs driving spontaneous (e.g., VAMP7 and vti1a) or evoked asynchronous (e.g., VAMP4) release integrates and corroborates existing data from functional and proteomic studies and implies that at least some complement of non-canonical SNAREs resident on synaptic vesicles function in neurotransmission. Here, we discuss the specific roles in neurotransmission of proteins homologous to each member of the classical neuronal SNARE complex consisting of synaptobrevin2, syntaxin-1, and SNAP-25.
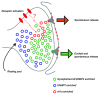
Figures

Similar articles
-
VAMP4 Maintains a Ca2+-Sensitive Pool of Spontaneously Recycling Synaptic Vesicles.J Neurosci. 2020 Jul 8;40(28):5389-5401. doi: 10.1523/JNEUROSCI.2386-19.2020. Epub 2020 Jun 12. J Neurosci. 2020. PMID: 32532887 Free PMC article.
-
Vesicle transport through interaction with t-SNAREs 1a (Vti1a)'s roles in neurons.Heliyon. 2020 Aug 1;6(8):e04600. doi: 10.1016/j.heliyon.2020.e04600. eCollection 2020 Aug. Heliyon. 2020. PMID: 32775753 Free PMC article. Review.
-
The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling.J Cell Biol. 1995 Feb;128(4):637-45. doi: 10.1083/jcb.128.4.637. J Cell Biol. 1995. PMID: 7860636 Free PMC article.
-
The t-SNARE syntaxin is sufficient for spontaneous fusion of synaptic vesicles to planar membranes.Cell Biol Int. 2000;24(11):809-18. doi: 10.1006/cbir.2000.0631. Cell Biol Int. 2000. PMID: 11067766
-
SNAREs in neurons--beyond synaptic vesicle exocytosis (Review).Mol Membr Biol. 2006 Sep-Oct;23(5):377-84. doi: 10.1080/09687860600776734. Mol Membr Biol. 2006. PMID: 17060155 Review.
Cited by
-
Dynamics of the mouse brain cortical synaptic proteome during postnatal brain development.Sci Rep. 2016 Oct 17;6:35456. doi: 10.1038/srep35456. Sci Rep. 2016. PMID: 27748445 Free PMC article.
-
The Sybtraps: control of synaptobrevin traffic by synaptophysin, α-synuclein and AP-180.Traffic. 2014 Mar;15(3):245-54. doi: 10.1111/tra.12140. Epub 2013 Dec 16. Traffic. 2014. PMID: 24279465 Free PMC article. Review.
-
Gβγ SNARE Interactions and Their Behavioral Effects.Neurochem Res. 2019 Mar;44(3):636-649. doi: 10.1007/s11064-018-2531-x. Epub 2018 May 11. Neurochem Res. 2019. PMID: 29752624 Free PMC article. Review.
-
Presynaptic origins of distinct modes of neurotransmitter release.Curr Opin Neurobiol. 2018 Aug;51:119-126. doi: 10.1016/j.conb.2018.03.005. Epub 2018 Mar 26. Curr Opin Neurobiol. 2018. PMID: 29597140 Free PMC article. Review.
-
Molecular underpinnings of synaptic vesicle pool heterogeneity.Traffic. 2015 Apr;16(4):338-64. doi: 10.1111/tra.12262. Traffic. 2015. PMID: 25620674 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
