Chlamydial infection induces host cytokinesis failure at abscission
- PMID: 22646503
- PMCID: PMC3443326
- DOI: 10.1111/j.1462-5822.2012.01820.x
Chlamydial infection induces host cytokinesis failure at abscission
Abstract
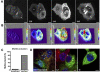
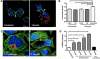
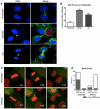
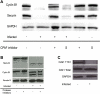
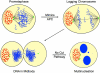
Chlamydia trachomatis is an obligate intracellular bacteria and the infectious agent responsible for the sexually transmitted disease Chlamydia. Infection with Chlamydia can lead to serious health sequelae such as pelvic inflammatory disease and reproductive tract scarring contributing to infertility and ectopic pregnancies. Additionally, chlamydial infections have been epidemiologically linked to cervical cancer in patients with a prior human papilomavirus (HPV) infection. Chlamydial infection of cultured cells causes multinucleation, a potential pathway for chromosomal instability. Two mechanisms that are known to initiate multinucleation are cell fusion and cytokinesis failure. This study demonstrates that multinucleation of the host cell by Chlamydia is entirely due to cytokinesis failure. Moreover, cytokinesis failure is due in part to the chlamydial effector CPAF acting as an anaphase promoting complex mimic causing cells to exit mitosis with unaligned and unattached chromosomes. These lagging and missegregated chromosomes inhibit cytokinesis by blocking abscission, the final stage of cytokinesis.
© 2012 Blackwell Publishing Ltd.
Figures

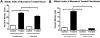

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

