Pharmacological comparison of novel synthetic fenamate analogues with econazole and 2-APB on the inhibition of TRPM2 channels
- PMID: 22646516
- PMCID: PMC3504990
- DOI: 10.1111/j.1476-5381.2012.02058.x
Pharmacological comparison of novel synthetic fenamate analogues with econazole and 2-APB on the inhibition of TRPM2 channels
Abstract
Background and purpose: Fenamate analogues, econazole and 2-aminoethoxydiphenyl borate (2-APB) are inhibitors of transient receptor potential melastatin 2 (TRPM2) channels and are used as research tools. However, these compounds have different chemical structures and therapeutic applications. Here we have investigated the pharmacological profile of TRPM2 channels by application of newly synthesized fenamate analogues and the existing channel blockers.
Experimental approach: Human TRPM2 channels in tetracycline-regulated pcDNA4/TO vectors were transfected into HEK293 T-REx cells and the expression was induced by tetracycline. Whole cell currents were recorded by patch-clamp techniques. Ca(2+) influx or release was monitored by fluorometry.
Key results: Flufenamic acid (FFA), mefenamic acid (MFA) and niflumic acid (NFA) concentration-dependently inhibited TRPM2 current with potency order FFA > MFA = NFA. Modification of the 2-phenylamino ring by substitution of the trifluoromethyl group in FFA with -CH(3), -F, -CF(3), -OCH(3), -OCH(2)CH(3), -COOH, and -NO(2) at various positions, reduced channel blocking potency. The conservative substitution of 3-CF(3) in FFA by -CH(3) (3-MFA), however, gave the most potent fenamate analogue with an IC(50) of 76 µM, comparable to that of FFA, but unlike FFA, had no effect on Ca(2+) release. 3-MFA and FFA inhibited the channel intracellularly. Econazole and 2-APB showed non-selectivity by altering cytosolic Ca(2+) movement. Econazole also evoked a non-selective current.
Conclusion and implications: The fenamate analogue 3-MFA was more selective than other TRPM2 channel blockers. FFA, 2-APB and econazole should be used with caution as TRPM2 channel blockers, as these compounds can interfere with intracellular Ca(2+) movement.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
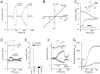




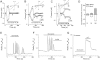
References
-
- Chang HT, Liu CS, Chou CT, Hsieh CH, Chang CH, Chen WC, et al. Econazole induces increases in free intracellular Ca2+ concentrations in human osteosarcoma cells. Hum Exp Toxicol. 2005;24:453–458. - PubMed
-
- Chien JM, Huang CC, Cheng HH, Lin KL, Chen WC, Tseng PL, et al. Econazole-evoked [Ca2+]i rise and non-Ca2+-triggered cell death in rabbit corneal epithelial cells (SIRC) J Recept Signal Transduct Res. 2008;28:567–579. - PubMed
-
- Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, et al. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem. 2005;95:715–723. - PubMed
-
- Foster RR, Zadeh MA, Welsh GI, Satchell SC, Ye Y, Mathieson PW, et al. Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells. Cell Calcium. 2009;45:384–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

