Role of small conductance calcium-activated potassium channels expressed in PVN in regulating sympathetic nerve activity and arterial blood pressure in rats
- PMID: 22647293
- PMCID: PMC3423990
- DOI: 10.1152/ajpregu.00114.2012
Role of small conductance calcium-activated potassium channels expressed in PVN in regulating sympathetic nerve activity and arterial blood pressure in rats
Abstract
Small conductance Ca(2+)-activated K(+) (SK) channels regulate membrane properties of rostral ventrolateral medulla (RVLM) projecting hypothalamic paraventricular nucleus (PVN) neurons and inhibition of SK channels increases in vitro excitability. Here, we determined in vivo the role of PVN SK channels in regulating sympathetic nerve activity (SNA) and mean arterial pressure (MAP). In anesthetized rats, bilateral PVN microinjection of SK channel blocker with peptide apamin (0, 0.125, 1.25, 3.75, 12.5, and 25 pmol) increased splanchnic SNA (SSNA), renal SNA (RSNA), MAP, and heart rate (HR) in a dose-dependent manner. Maximum increases in SSNA, RSNA, MAP, and HR elicited by apamin (12.5 pmol, n = 7) were 330 ± 40% (P < 0.01), 271 ± 40% (P < 0.01), 29 ± 4 mmHg (P < 0.01), and 34 ± 9 beats/min (P < 0.01), respectively. PVN injection of the nonpeptide SK channel blocker UCL1684 (250 pmol, n = 7) significantly increased SSNA (P < 0.05), RSNA (P < 0.05), MAP (P < 0.05), and HR (P < 0.05). Neither apamin injected outside the PVN (12.5 pmol, n = 6) nor peripheral administration of the same dose of apamin (12.5 pmol, n = 5) evoked any significant changes in the recorded variables. PVN-injected SK channel enhancer 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazol-2-one (DCEBIO, 5 nmol, n = 4) or N-cyclohexyl-N-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidin]amine (CyPPA, 5 nmol, n = 6) did not significantly alter the SSNA, RSNA, MAP, and HR. Western blot and RT-PCR analysis of punched PVN tissue showed abundant expression of SK1-3 channels. We conclude that SK channels expressed in the PVN play an important role in the regulation of sympathetic outflow and cardiovascular function.
Figures
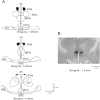
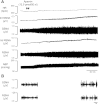


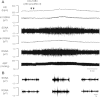
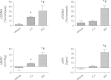

References
-
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74: 245–269, 2012 - PubMed
-
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995 - PubMed
-
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 77: 3396–3400, 1997 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

