A doxycycline-dependent human immunodeficiency virus type 1 replicates in vivo without inducing CD4+ T-cell depletion
- PMID: 22647372
- PMCID: PMC3542129
- DOI: 10.1099/vir.0.042796-0
A doxycycline-dependent human immunodeficiency virus type 1 replicates in vivo without inducing CD4+ T-cell depletion
Abstract
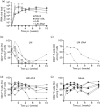
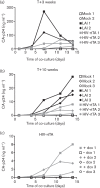
A novel genetic approach for the control of virus replication was used for the design of a conditionally replicating human immunodeficiency virus (HIV) variant, HIV-rtTA. HIV-rtTA gene expression and virus replication are strictly dependent on the presence of a non-toxic effector molecule, doxycycline (dox), and thus can be turned on and off at will in a graded and reversible manner. The in vivo replication capacity, pathogenicity and genetic stability of this HIV-rtTA variant were evaluated in a humanized mouse model of haematopoiesis that harbours lymphoid and myeloid components of the human immune system (HIS). Infection of dox-fed BALB Rag/γc HIS (BRG-HIS) mice with HIV-rtTA led to the establishment of a productive infection without CD4(+) T-cell depletion. The virus did not show any sign of escape from dox control for up to 10 weeks after the onset of infection. No reversion towards a functional Tat-transactivating responsive (TAR) RNA element axis was observed, confirming the genetic stability of the HIV-rtTA variant in vivo. These results demonstrate the proof of concept that HIV-rtTA replicates efficiently in vivo. HIV-rtTA is a promising tool for fundamental research to study virus-host interactions in vivo in a controlled fashion.
Figures



Similar articles
-
Conditionally replicating HIV and SIV variants.Virus Res. 2016 May 2;216:66-75. doi: 10.1016/j.virusres.2015.05.004. Epub 2015 May 15. Virus Res. 2016. PMID: 25982510 Review.
-
Optimization of the doxycycline-dependent simian immunodeficiency virus through in vitro evolution.Retrovirology. 2008 Jun 5;5:44. doi: 10.1186/1742-4690-5-44. Retrovirology. 2008. PMID: 18533993 Free PMC article.
-
Construction of Nef-positive doxycycline-dependent HIV-1 variants using bicistronic expression elements.Virology. 2016 Jan 15;488:96-107. doi: 10.1016/j.virol.2015.11.004. Epub 2015 Nov 23. Virology. 2016. PMID: 26615334
-
Modification of the Tet-On regulatory system prevents the conditional-live HIV-1 variant from losing doxycycline-control.Retrovirology. 2006 Nov 9;3:82. doi: 10.1186/1742-4690-3-82. Retrovirology. 2006. PMID: 17094796 Free PMC article.
-
Conditional virus replication as an approach to a safe live attenuated human immunodeficiency virus vaccine.J Neurovirol. 2002 Dec;8 Suppl 2:134-7. doi: 10.1080/13550280290101102. J Neurovirol. 2002. PMID: 12491165 Review.
Cited by
-
Tet-On Systems For Doxycycline-inducible Gene Expression.Curr Gene Ther. 2016;16(3):156-67. doi: 10.2174/1566523216666160524144041. Curr Gene Ther. 2016. PMID: 27216914 Free PMC article. Review.
-
Strategies for the Construction of Mouse Models With Humanized Immune System and Evaluation of Tumor Immune Checkpoint Inhibitor Therapy.Front Oncol. 2021 Apr 29;11:673199. doi: 10.3389/fonc.2021.673199. eCollection 2021. Front Oncol. 2021. PMID: 33996603 Free PMC article. Review.
-
Humanized Mice for Live-Attenuated Vaccine Research: From Unmet Potential to New Promises.Vaccines (Basel). 2020 Jan 21;8(1):36. doi: 10.3390/vaccines8010036. Vaccines (Basel). 2020. PMID: 31973073 Free PMC article. Review.
-
New challenges in modern vaccinology.BMC Immunol. 2015 Mar 26;16:18. doi: 10.1186/s12865-015-0075-2. BMC Immunol. 2015. PMID: 25879661 Free PMC article. Review.
References
-
- An D. S., Poon B., Fang R. H. T., Weijer K., Blom B., Spits H., Chen I. S. Y., Uittenbogaart C. H. (2007). Use of a novel chimeric mouse model with a functionally active human immune system to study human immunodeficiency virus type 1 infection. Clin Vaccine Immunol 14, 391–396 10.1128/CVI.00403-06 - DOI - PMC - PubMed
-
- Baenziger S., Tussiwand R., Schlaepfer E., Mazzucchelli L., Heikenwalder M., Kurrer M. O., Behnke S., Frey J., Oxenius A. & other authors (2006). Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γ c−/− mice. Proc Natl Acad Sci U S A 103, 15951–15956 10.1073/pnas.0604493103 - DOI - PMC - PubMed
-
- Becker P. D., Legrand N., van Geelen C. M., Noerder M., Huntington N. D., Lim A., Yasuda E., Diehl S. A., Scheeren F. A. & other authors (2010). Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS ONE 5, e13137 10.1371/journal.pone.0013137 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

