Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer's disease
- PMID: 22647384
- PMCID: PMC3506930
- DOI: 10.1186/alzrt118
Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer's disease
Abstract
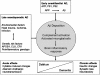
Neuropathological studies have revealed the presence of a broad variety of inflammation-related proteins (complement factors, acute-phase proteins, pro-inflammatory cytokines) in Alzheimer's disease (AD) brains. These constituents of innate immunity are involved in several crucial pathogenic events of the underlying pathological cascade in AD, and recent studies have shown that innate immunity is involved in the etiology of late-onset AD. Genome-wide association studies have demonstrated gene loci that are linked to the complement system. Neuropathological and experimental studies indicate that fibrillar amyloid-β (Aβ) can activate the innate immunity-related CD14 and Toll-like receptor signaling pathways of glial cells for pro-inflammatory cytokine production. The production capacity of this pathway is under genetic control and offspring with a parental history of late-onset AD have a higher production capacity for pro-inflammatory cytokines. The activation of microglia by fibrillar Aβ deposits in the early preclinical stages of AD can make the brain susceptible later on for a second immune challenge leading to enhanced production of pro-inflammatory cytokines. An example of a second immune challenge could be systemic inflammation in patients with preclinical AD. Prospective epidemiological studies show that elevated serum levels of acute phase reactants can be considered as a risk factor for AD. Clinical studies suggest that peripheral inflammation increases the risk of dementia, especially in patients with preexistent cognitive impairment, and accelerates further deterioration in demented patients. The view that peripheral inflammation can increase the risk of dementia in older people provides scope for prevention.
Figures
References
-
- Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJM, van Gool WA, Hoozemans JJM. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm. 2006;113:1685–1695. - PubMed
-
- Heneka MT, O'Banion MK, Terwel D, Kummer MP. Neuroinflamatory processes in Alzheimer's disease. J Neural Transm. 2010;117:919–947. - PubMed
-
- Hoozemans JJM, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int J Dev Neurosci. 2006;24:157–165. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

