A gene-protein assay for human epidermal growth factor receptor 2 (HER2): brightfield tricolor visualization of HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17) in formalin-fixed, paraffin-embedded breast cancer tissue sections
- PMID: 22647525
- PMCID: PMC3487810
- DOI: 10.1186/1746-1596-7-60
A gene-protein assay for human epidermal growth factor receptor 2 (HER2): brightfield tricolor visualization of HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17) in formalin-fixed, paraffin-embedded breast cancer tissue sections
Abstract
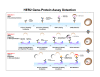
Background: The eligibility of breast cancer patients for human epidermal growth factor receptor 2 (HER2)-directed therapies is determined by the HER2 gene amplification and/or HER2 protein overexpression status of the breast tumor as determined by in situ hybridization (ISH) or immunohistochemistry (IHC), respectively. Our objective was to combine the US Food and Drug Administration (FDA)-approved HER2 & chromosome 17 centromere (CEN17) brightfield ISH (BISH) and HER2 IHC assays into a single automated HER2 gene-protein assay allowing simultaneous detection of all three targets in a single tissue section.
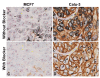
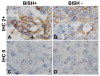
Methods: The HER2 gene-protein assay was optimized using formalin-fixed, paraffin-embedded (FFPE) samples of the xenograft tumors MCF7 [HER2 negative (non-amplified gene, protein negative)] and Calu-3 [HER2 positive (amplified gene, protein positive)]. HER2 IHC was performed using a rabbit monoclonal anti-HER2 antibody (clone 4B5) and a conventional 3,3'-diaminobenzidine IHC detection. The HER2 & CEN17 BISH signals were visualized using horseradish peroxidase-based silver and alkaline phosphatase-based red detection systems, respectively with a cocktail of 2,4-dinitrophenyl-labeled HER2 and digoxigenin-labeled CEN17 probes. The performance of the gene-protein assay on tissue microarray slides containing 189 randomly selected FFPE clinical breast cancer tissue cores was compared to that of the separate HER2 IHC and HER2 & CEN17 BISH assays.
Results: HER2 protein detection was optimal when the HER2 IHC protocol was used before (rather than after) the BISH protocol. The sequential use of HER2 IHC and HER2 & CEN17 BISH detection steps on FFPE xenograft tumor sections appropriately co-localized the HER2 protein, HER2 gene, and CEN17 signals after mitigating the silver background staining by using a naphthol phosphate-containing hybridization buffer for the hybridization step. The HER2 protein and HER2 gene status obtained using the multiplex HER2 gene-protein assay demonstrated high concordance with those obtained using the separate HER2 IHC and HER2 & CEN17 BISH assays, respectively.
Conclusions: We have developed a protocol that allows simultaneous visualization of the HER2 IHC and HER2 & CEN17 BISH targets. This automated protocol facilitated the determination of HER2 protein and HER2 gene status in randomly selected breast cancer samples, particularly in cases that were equivocal or exhibited tumor heterogeneity. The HER2 gene-protein assay produced results virtually equivalent to those of the single FDA-approved HER2 IHC and HER2 & CEN17 BISH assays.
Virtual slides: The virtual slides for this article can be found here: http://www.diagnosticpathology.diagnomx.eu/vs/2041964038705297.
Figures


Similar articles
-
Droplet digital polymerase chain reaction detection of HER2 amplification in formalin fixed paraffin embedded breast and gastric carcinoma samples.Exp Mol Pathol. 2016 Apr;100(2):287-93. doi: 10.1016/j.yexmp.2015.11.027. Epub 2015 Nov 25. Exp Mol Pathol. 2016. PMID: 26626802
-
Quantitative real-time polymerase chain reaction is an alternative method for the detection of HER-2 amplification in formalin-fixed paraffin-embedded breast cancer samples.Int J Clin Exp Pathol. 2015 Sep 1;8(9):10565-74. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617766 Free PMC article.
-
Gene protein detection platform--a comparison of a new human epidermal growth factor receptor 2 assay with conventional immunohistochemistry and fluorescence in situ hybridization platforms.Ann Diagn Pathol. 2015 Aug;19(4):203-10. doi: 10.1016/j.anndiagpath.2015.04.002. Epub 2015 Apr 7. Ann Diagn Pathol. 2015. PMID: 25921313
-
The assessment of HER2 status in breast cancer: the past, the present, and the future.Pathol Int. 2016 Jun;66(6):313-24. doi: 10.1111/pin.12407. Epub 2016 Apr 7. Pathol Int. 2016. PMID: 27061008 Review.
-
Discordance Between Immunohistochemistry and In Situ Hybridization to Detect HER2 Overexpression/Gene Amplification in Breast Cancer in the Modern Age: A Single Institution Experience and Pooled Literature Review Study.Clin Breast Cancer. 2022 Jan;22(1):e123-e133. doi: 10.1016/j.clbc.2021.05.004. Epub 2021 May 17. Clin Breast Cancer. 2022. PMID: 34120846 Review.
Cited by
-
pHLIP-fused PD-L1 engages avelumab to elicit NK cytotoxicity under acidic conditions.Heliyon. 2024 May 3;10(9):e30551. doi: 10.1016/j.heliyon.2024.e30551. eCollection 2024 May 15. Heliyon. 2024. PMID: 38756565 Free PMC article.
-
Assessment of MYC/PTEN Status by Gene-Protein Assay in Grade Group 2 Prostate Biopsies.J Mol Diagn. 2021 Aug;23(8):1030-1041. doi: 10.1016/j.jmoldx.2021.05.006. Epub 2021 May 29. J Mol Diagn. 2021. PMID: 34062284 Free PMC article.
-
Deciphering HER2 Breast Cancer Disease: Biological and Clinical Implications.Front Oncol. 2019 Oct 29;9:1124. doi: 10.3389/fonc.2019.01124. eCollection 2019. Front Oncol. 2019. PMID: 31737566 Free PMC article. Review.
-
HER2-low metastases of HER2-negative primary tumors: a single institution analysis of intertumoral and internodal heterogeneity in node-positive breast cancer.Front Oncol. 2023 Jul 7;13:1167567. doi: 10.3389/fonc.2023.1167567. eCollection 2023. Front Oncol. 2023. PMID: 37483511 Free PMC article.
-
Intratumoral Estrogen Receptor Heterogeneity of Expression in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer as Evaluated by a Brightfield Multiplex Assay.J Histochem Cytochem. 2019 Aug;67(8):563-574. doi: 10.1369/0022155419856862. Epub 2019 Jun 11. J Histochem Cytochem. 2019. PMID: 31184528 Free PMC article.
References
-
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. - PubMed
-
- Sauter G, Lee J, Barlett JMS, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1324–1333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

