Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization
- PMID: 22647600
- PMCID: PMC3386059
- DOI: 10.1073/pnas.1119038109
Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization
Abstract
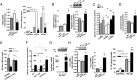
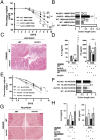
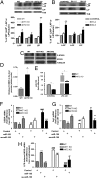
Activated macrophages are described as classically activated or M1 type and alternatively activated or M2 type, depending on their response to proinflammatory stimuli and the expression of genetic markers including iNOS, arginase1, Ym1, and Fizz1. Here we report that Akt kinases differentially contribute to macrophage polarization, with Akt1 ablation giving rise to an M1 and Akt2 ablation resulting in an M2 phenotype. Accordingly, Akt2(-/-) mice were more resistant to LPS-induced endotoxin shock and to dextran sulfate sodium (DSS)-induced colitis than wild-type mice, whereas Akt1(-/-) mice were more sensitive. Cell depletion and reconstitution experiments in a DSS-induced colitis model confirmed that the effect was macrophage-dependent. Gene-silencing studies showed that the M2 phenotype of Akt2(-/-) macrophages was cell autonomous. The microRNA miR-155, whose expression was repressed in naive and in LPS-stimulated Akt2(-/-) macrophages, and its target C/EBPβ appear to play a key role in this process. C/EBPβ, a hallmark of M2 macrophages that regulates Arg1, was up-regulated upon Akt2 ablation or silencing. Overexpression or silencing of miR-155 confirmed its central role in Akt isoform-dependent M1/M2 polarization of macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. - PubMed
-
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. - PubMed
-
- Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

