Functional role of sodium glucose transporter in high glucose-mediated angiotensin type 1 receptor downregulation in human proximal tubule cells
- PMID: 22647632
- PMCID: PMC3468487
- DOI: 10.1152/ajprenal.00651.2011
Functional role of sodium glucose transporter in high glucose-mediated angiotensin type 1 receptor downregulation in human proximal tubule cells
Abstract
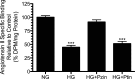
Previously, we have demonstrated human angiotensin type 1 receptor (hAT(1)R) promoter architecture with regard to the effect of high glucose (25 mM)-mediated transcriptional repression in human proximal tubule epithelial cells (hPTEC; Thomas BE, Thekkumkara TJ. Mol Biol Cell 15: 4347-4355, 2004). In the present study, we investigated the role of glucose transporters in high glucose-mediated hAT(1)R repression in primary hPTEC. Cells were exposed to normal glucose (5.5 mM) and high glucose (25 mM), followed by determination of hyperglycemia-mediated changes in receptor expression and glucose transporter activity. Exposure of cells to high glucose resulted in downregulation of ANG II binding (4,034 ± 163.3 to 1,360 ± 154.3 dpm/mg protein) and hAT(1)R mRNA expression (reduced 60.6 ± 4.643%) at 48 h. Under similar conditions, we observed a significant increase in glucose uptake (influx) in cells exposed to hyperglycemia. Our data indicated that the magnitude of glucose influx is concentration and time dependent. In euglycemic cells, inhibiting sodium-glucose cotransporters (SGLTs) with phlorizin and facilitative glucose transporters (GLUTs) with phloretin decreased glucose influx by 28.57 ± 0.9123 and 54.33 ± 1.202%, respectively. However, inhibiting SGLTs in cells under hyperglycemic conditions decreased glucose influx by 53.67 ± 2.906%, while GLUT-mediated glucose uptake remained unaltered (57.67 ± 3.180%). Furthermore, pretreating cells with an SGLT inhibitor reversed high glucose-mediated downregulation of the hAT(1)R, suggesting an involvement of SGLT in high glucose-mediated hAT(1)R repression. Our results suggest that in hPTEC, hyperglycemia-induced hAT(1)R downregulation is largely mediated through SGLT-dependent glucose influx. As ANG II is an important modulator of hPTEC transcellular sodium reabsorption and function, glucose-mediated changes in hAT(1)R gene expression may participate in the pathogenesis of diabetic renal disease.
Figures






References
-
- Baer PC, Nockher WA, Haase W, Scherberich JE. Isolation of proximal and distal tubule cells from human kidney by immuno-magnetic separation. Kidney Int 52: 1321–1331, 1997 - PubMed
-
- Bagby SP, LeBard LS, Luo Z, Speth RC, Ogden BE, Corless CL. Angiotensin II type 1 and 2 receptors in conduit arteries of normal developing microswine. Arterioscler Thromb Vasc Biol 22: 1113–1121, 2002 - PubMed
-
- Becker BN, Kondo S, Cheng HF, Harris RC. Effect of glucose, pyruvate, and insulin on type 1 angiotensin II receptor expression in SV40-immortalized rabbit proximal tubule epithelial cells. Kidney Int 52: 87–92, 1997 - PubMed
-
- Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care 13: 198–208, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

