Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats
- PMID: 22647647
- PMCID: PMC3517515
- DOI: 10.1186/1756-6606-5-18
Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats
Abstract
Background: Spinal glia, particularly microglia and astrocytes, are of the utmost importance in the development and maintenance of chronic pain. A recent study from our laboratory revealed that gabapentin, a recommended first-line treatment for multiple neuropathic conditions, could also efficiently antagonize thermal hyperalgesia evoked by complete Freund's adjuvant (CFA)-induced monoarthritis (MA). In the present study, we investigated whether the spinal glia are involved in the anti-hyperalgesic effect of gabapentin and how this event occurs.
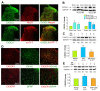
Results: Unilateral intra-articular injection of CFA produced a robust activation of microglia and astrocytes. These cells exhibited large cell bodies, thick processes and increases in the ionized calcium binding adapter molecule 1 (Iba-1, a microglial marker) or the glia fibrillary acidic protein (GFAP, an astrocytic marker). These cells also displayed immunoreactive signals, and an upregulation of the voltage-gated calcium channels (VGCCs) α2/δ-1 subunit, CX3CL1 and CX3CR1 expression levels in the spinal cord. These changes were associated with the development of thermal hyperalgesia. Immunofluorescence staining showed that VGCC α2/δ-1 subunit, a proposed gabapentin target of action, was widely distributed in primary afferent fibers terminals and dorsal horn neurons. CX3CL1, a potential trigger to activate microglia, colocalized with VGCC α2/δ-1 subunits in the spinal dorsal horn. However, its receptor CX3CR1 was mainly expressed in the spinal microglia. Multiple intraperitoneal (i.p.) gabapentin injections (100 mg/kg, once daily for 4 days with the first injection 60 min before intra-articular CFA) suppressed the activation of spinal microglia, downregulated spinal VGCC α2/δ-1 subunits decreased CX3CL1 levels and blocked the development of thermal hyperalgesia in MA rats.
Conclusions: Here we provide the first evidence that gabapentin diminishes CX3CL1 signaling and spinal microglia activation induced by joint inflammation. We also show that the VGCC α2/δ-1 subunits might be involved in these events.
Figures




Similar articles
-
Dexmedetomidine blocks thermal hyperalgesia and spinal glial activation in rat model of monoarthritis.Acta Pharmacol Sin. 2010 May;31(5):523-30. doi: 10.1038/aps.2010.32. Epub 2010 Apr 5. Acta Pharmacol Sin. 2010. PMID: 20364156 Free PMC article.
-
Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain.J Pain. 2005 Jul;6(7):434-8. doi: 10.1016/j.jpain.2005.02.001. J Pain. 2005. PMID: 15993821
-
Gabapentin decreases microglial cells and reverses bilateral hyperalgesia and allodynia in rats with chronic myositis.Eur J Pharmacol. 2017 Mar 15;799:111-117. doi: 10.1016/j.ejphar.2017.02.012. Epub 2017 Feb 10. Eur J Pharmacol. 2017. PMID: 28192096
-
CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases.Pharmacol Ther. 2022 Mar;231:107989. doi: 10.1016/j.pharmthera.2021.107989. Epub 2021 Sep 4. Pharmacol Ther. 2022. PMID: 34492237 Review.
-
Chemokines and pain mechanisms.Brain Res Rev. 2009 Apr;60(1):125-34. doi: 10.1016/j.brainresrev.2008.12.002. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19146875 Free PMC article. Review.
Cited by
-
Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain.Pharmaceuticals (Basel). 2023 Jul 19;16(7):1023. doi: 10.3390/ph16071023. Pharmaceuticals (Basel). 2023. PMID: 37513935 Free PMC article.
-
Anti-nociceptive effect of dexmedetomidine in a rat model of monoarthritis via suppression of the TLR4/NF-κB p65 pathway.Exp Ther Med. 2017 Nov;14(5):4910-4918. doi: 10.3892/etm.2017.5196. Epub 2017 Sep 22. Exp Ther Med. 2017. PMID: 29201195 Free PMC article.
-
Acute and Chronic Ethanol Effects during Adolescence on Neuroimmune Responses: Consequences and Potential Pharmacologic Interventions.Cells. 2023 May 18;12(10):1423. doi: 10.3390/cells12101423. Cells. 2023. PMID: 37408257 Free PMC article. Review.
-
The therapeutic potential of targeting chemokine signalling in the treatment of chronic pain.J Neurochem. 2017 May;141(4):520-531. doi: 10.1111/jnc.13927. Epub 2017 Feb 24. J Neurochem. 2017. PMID: 27973687 Free PMC article. Review.
-
Gabapentin-Friend or foe?Pain Pract. 2023 Jan;23(1):63-69. doi: 10.1111/papr.13165. Epub 2022 Oct 27. Pain Pract. 2023. PMID: 36300903 Free PMC article. Review.
References
-
- Twining CM, Sloane EM, Schoeniger DK, Milligan ED, Martin D, Marsh H, Maier SF, Watkins LR. Activation of the spinal cord complement cascade might contribute to mechanical allodynia induced by three animal models of spinal sensitization. J Pain. 2005;6:174–183. doi: 10.1016/j.jpain.2004.11.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

