Regulation of virus neutralization and the persistent fraction by TRIM21
- PMID: 22647693
- PMCID: PMC3421726
- DOI: 10.1128/JVI.00728-12
Regulation of virus neutralization and the persistent fraction by TRIM21
Abstract
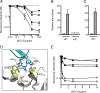
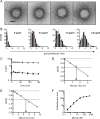
Despite a central role in immunity, antibody neutralization of virus infection is poorly understood. Here we show how the neutralization and persistence of adenovirus type 5, a prevalent nonenveloped human virus, are dependent upon the intracellular antibody receptor TRIM21. Cells with insufficient amounts of TRIM21 are readily infected, even at saturating concentrations of neutralizing antibody. Conversely, high TRIM21 expression levels decrease the persistent fraction of the infecting virus and allows neutralization by as few as 1.6 antibody molecules per virus. The direct interaction between TRIM21 and neutralizing antibody is essential, as single-point mutations within the TRIM21-binding site in the Fc region of a potently neutralizing antibody impair neutralization. However, infection at high multiplicity can saturate TRIM21 and overcome neutralization. These results provide insight into the mechanism and importance of a newly discovered, effector-driven process of antibody neutralization of nonenveloped viruses.
Figures


References
-
- Booy FP, Roden RB, Greenstone HL, Schiller JT, Trus BL. 1998. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 A resolution. J. Mol. Biol. 281:95–106 - PubMed
-
- Burton DR, Saphire EO, Parren PW. 2001. A model for neutralization of viruses based on antibody coating of the virion surface. Curr. Top. Microbiol. Immunol. 260:109–143 - PubMed
-
- de Martin R, Raidl M, Hofer E, Binder BR. 1997. Adenovirus-mediated expression of green fluorescent protein. Gene Ther. 4:493–495 - PubMed
-
- Dimmock NJ. 1984. Mechanisms of neutralization of animal viruses. J. Gen. Virol. 65(Pt 6):1015–1022 - PubMed
-
- Dimmock NJ. 1995. Update on the neutralization of animal viruses. Rev. Med. Virol. 5:165–179
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

