The clathrin adaptor Dab2 recruits EH domain scaffold proteins to regulate integrin β1 endocytosis
- PMID: 22648170
- PMCID: PMC3408417
- DOI: 10.1091/mbc.E11-12-1007
The clathrin adaptor Dab2 recruits EH domain scaffold proteins to regulate integrin β1 endocytosis
Abstract
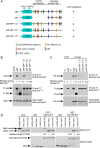
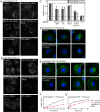
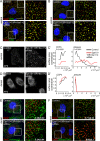
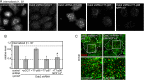
Endocytic adaptor proteins facilitate cargo recruitment and clathrin-coated pit nucleation. The prototypical clathrin adaptor AP2 mediates cargo recruitment, maturation, and scission of the pit by binding cargo, clathrin, and accessory proteins, including the Eps-homology (EH) domain proteins Eps15 and intersectin. However, clathrin-mediated endocytosis of some cargoes proceeds efficiently in AP2-depleted cells. We found that Dab2, another endocytic adaptor, also binds to Eps15 and intersectin. Depletion of EH domain proteins altered the number and size of clathrin structures and impaired the endocytosis of the Dab2- and AP2-dependent cargoes, integrin β1 and transferrin receptor, respectively. To test the importance of Dab2 binding to EH domain proteins for endocytosis, we mutated the EH domain-binding sites. This mutant localized to clathrin structures with integrin β1, AP2, and reduced amounts of Eps15. Of interest, although integrin β1 endocytosis was impaired, transferrin receptor internalization was unaffected. Surprisingly, whereas clathrin structures contain both Dab2 and AP2, integrin β1 and transferrin localize in separate pits. These data suggest that Dab2-mediated recruitment of EH domain proteins selectively drives the internalization of the Dab2 cargo, integrin β1. We propose that adaptors may need to be bound to their cargo to regulate EH domain proteins and internalize efficiently.
Figures

Similar articles
-
FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis.Mol Biol Cell. 2012 Apr;23(7):1330-42. doi: 10.1091/mbc.E11-09-0812. Epub 2012 Feb 9. Mol Biol Cell. 2012. PMID: 22323290 Free PMC article.
-
The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH.J Cell Sci. 2006 Oct 15;119(Pt 20):4235-46. doi: 10.1242/jcs.03217. Epub 2006 Sep 19. J Cell Sci. 2006. PMID: 16984970
-
A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors.Mol Biol Cell. 2006 Oct;17(10):4300-17. doi: 10.1091/mbc.e06-05-0421. Epub 2006 Jul 26. Mol Biol Cell. 2006. PMID: 16870701 Free PMC article.
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
-
Evolving models for assembling and shaping clathrin-coated pits.J Cell Biol. 2020 Sep 7;219(9):e202005126. doi: 10.1083/jcb.202005126. J Cell Biol. 2020. PMID: 32770195 Free PMC article. Review.
Cited by
-
An autoinhibitory clamp of actin assembly constrains and directs synaptic endocytosis.Elife. 2021 Jul 29;10:e69597. doi: 10.7554/eLife.69597. Elife. 2021. PMID: 34324418 Free PMC article.
-
Antagonistic control of active surface integrins by myotubularin and phosphatidylinositol 3-kinase C2β in a myotubular myopathy model.Proc Natl Acad Sci U S A. 2022 Oct 4;119(40):e2202236119. doi: 10.1073/pnas.2202236119. Epub 2022 Sep 26. Proc Natl Acad Sci U S A. 2022. PMID: 36161941 Free PMC article.
-
Endocytic Adaptor Proteins in Health and Disease: Lessons from Model Organisms and Human Mutations.Cells. 2019 Oct 29;8(11):1345. doi: 10.3390/cells8111345. Cells. 2019. PMID: 31671891 Free PMC article. Review.
-
A nanobody-based molecular toolkit provides new mechanistic insight into clathrin-coat initiation.Elife. 2019 Apr 30;8:e41768. doi: 10.7554/eLife.41768. Elife. 2019. PMID: 31038455 Free PMC article.
-
Integrin trafficking in cells and tissues.Nat Cell Biol. 2019 Feb;21(2):122-132. doi: 10.1038/s41556-018-0223-z. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602723 Free PMC article. Review.
References
-
- Cao TT, Mays RW, von Zastrow M. Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. J Biol Chem. 1998;273:24592–24602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

