Performance of methods for handling missing categorical covariate data in population pharmacokinetic analyses
- PMID: 22648902
- PMCID: PMC3385822
- DOI: 10.1208/s12248-012-9373-2
Performance of methods for handling missing categorical covariate data in population pharmacokinetic analyses
Abstract
In population pharmacokinetic analyses, missing categorical data are often encountered. We evaluated several methods of performing covariate analyses with partially missing categorical covariate data. Missing data methods consisted of discarding data (DROP), additional effect parameter for the group with missing data (EXTRA), and mixture methods in which the mixing probability was fixed to the observed fraction of categories (MIX(obs)), based on the likelihood of the concentration data (MIX(conc)), or combined likelihood of observed covariate data and concentration data (MIX(joint)). Simulations were implemented to study bias and imprecision of the methods in datasets with equal-sized and unbalanced category ratios for a binary covariate as well as datasets with non-random missingness (MNAR). Additionally, the performance and feasibility of implementation was assessed in two real datasets. At either low (10%) or high (50%) levels of missingness, all methods performed similarly well. Performance was similar for situations with unbalanced datasets (3:1 covariate distribution) and balanced datasets. In the MNAR scenario, the MIX methods showed a higher bias in the estimation of CL and covariate effect than EXTRA. All methods could be applied to real datasets, except DROP. All methods perform similarly at the studied levels of missingness, but the DROP and EXTRA methods provided less bias than the mixture methods in the case of MNAR. However, EXTRA was associated with inflated type I error rates of covariate selection, while DROP handled data inefficiently.
Figures
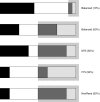


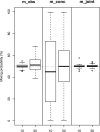


References
-
- Joerger M, Huitema ADR, van den Bongard D, Schellens JHM, Beijnen JH. Quantitative effect of gender, age, liver function, and body size on the population pharmacokinetics of Paclitaxel in patients with solid tumors. Clin Cancer Res. 2006;12(7 Pt 1):2150–7. doi: 10.1158/1078-0432.CCR-05-2069. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

