Structural insights into the assembly of large oligomeric signalosomes in the Toll-like receptor-interleukin-1 receptor superfamily
- PMID: 22649099
- PMCID: PMC3568634
- DOI: 10.1126/scisignal.2003124
Structural insights into the assembly of large oligomeric signalosomes in the Toll-like receptor-interleukin-1 receptor superfamily
Abstract
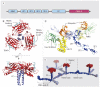
The Toll-like receptor (TLR)-interleukin 1 receptor (IL-1R) superfamily plays fundamentally important roles in innate immune and inflammatory responses. Structural studies have begun to show that upon ligand stimulation, TLRs and IL-1Rs assemble large oligomeric intracellular signaling complexes, or "signalosomes," to induce the activation of kinases and E3 ubiquitin ligases, leading eventually to the activation of the transcription factors that are responsible for the expression of genes whose products mediate immune and inflammatory responses. The different scaffolds identified by these structural studies provide a molecular foundation for understanding the formation of microscopically visible signaling clusters that have long been known to cell biologists. Here, we illustrate the potential mechanisms of step-by-step assembly from the membrane-proximal interactions to the more downstream events. Formation of large oligomeric signalosomes may help to establish a digital threshold response in TLR and IL-1R signaling.
Figures



References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nature reviews. Immunology. 2004;4:499–511. - PubMed
-
- Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. Journal of leukocyte biology. 2003;74:479–485. - PubMed
-
- Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Current opinion in immunology. 2003;15:396–401. - PubMed
-
- Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science (New York, N.Y.) 1997;278:1612–1615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

