Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma--a report from the Children's Oncology Group
- PMID: 22649136
- PMCID: PMC3434976
- DOI: 10.1200/JCO.2011.41.1819
Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma--a report from the Children's Oncology Group
Abstract
Purpose: In 1995, the Children's Cancer Group (CCG) opened a trial for patients with Hodgkin's lymphoma evaluating whether low-dose involved-field radiation therapy (IFRT) improved event-free survival (EFS) for patients achieving a complete response after chemotherapy. We present the long-term study outcome using final data through March 2007.
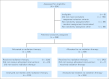
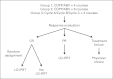
Patients and methods: Between January 1995 and December 1998, 826 eligible patients were enrolled onto CCG 5942. Four hundred ninety-eight patients achieving an initial complete response to chemotherapy were randomly assigned to receive IFRT or no further therapy. EFS and overall survival (OS) were assessed from the date of study entry or random assignment, as appropriate.
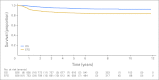
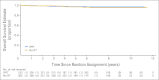
Results: Ten-year EFS and OS rates for the entire cohort were 83.5% and 92.5%, respectively. In an as-treated analysis for randomly assigned patients, the 10-year EFS and OS rates were 91.2% and 97.1%, respectively, for IFRT and 82.9% and 95.9%, respectively, for no further therapy. For EFS and OS comparisons, P = .004 and P = .50, respectively. Bulk disease, "B" symptoms, and nodular sclerosis histology were risk factors for inferior EFS.
Conclusion: With a median follow-up of 7.7 years, IFRT produced a statistically significant improvement in EFS but no improvement in OS. For individual patients, the relative risks of relapse versus late effects of IFRT must be considered. Patient and disease characteristics and early response assessment will aid in deciding which patients are most likely to benefit from IFRT.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Finding the balance in pediatric Hodgkin's lymphoma.J Clin Oncol. 2012 Sep 10;30(26):3158-9. doi: 10.1200/JCO.2012.42.6890. Epub 2012 May 29. J Clin Oncol. 2012. PMID: 22649142 No abstract available.
-
[Does involved field radiotherapy improve survival for children with Hodgkin's lymphoma in complete remission after chemotherapy?].Strahlenther Onkol. 2013 Apr;189(4):344-6. doi: 10.1007/s00066-013-0307-4. Strahlenther Onkol. 2013. PMID: 23417541 German. No abstract available.
References
-
- Tebbi CK, Mendenhall N, London WB, et al. Treatment of stage I, IIA, IIIA1 pediatric Hodgkin disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation: A Pediatric Oncology Group (POG) study. Pediatr Blood Cancer. 2006;46:198–202. - PubMed
-
- Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin's disease. J Clin Oncol. 2007;25:332–337. - PubMed
-
- Dörffel W, Lüders H, Rühl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin's disease in children and adolescents: Analysis and outlook. Klin Padiatr. 2003;215:139–145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

