Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657
- PMID: 22649152
- PMCID: PMC3434983
- DOI: 10.1200/JCO.2011.39.2779
Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657
Abstract
Purpose: Neoadjuvant chemotherapy for breast cancer provides critical information about tumor response; how best to leverage this for predicting recurrence-free survival (RFS) is not established. The I-SPY 1 TRIAL (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging and Molecular Analysis) was a multicenter breast cancer study integrating clinical, imaging, and genomic data to evaluate pathologic response, RFS, and their relationship and predictability based on tumor biomarkers.
Patients and methods: Eligible patients had tumors ≥ 3 cm and received neoadjuvant chemotherapy. We determined associations between pathologic complete response (pCR; defined as the absence of invasive cancer in breast and nodes) and RFS, overall and within receptor subsets.
Results: In 221 evaluable patients (median tumor size, 6.0 cm; median age, 49 years; 91% classified as poor risk on the basis of the 70-gene prognosis profile), 41% were hormone receptor (HR) negative, and 31% were human epidermal growth factor receptor 2 (HER2) positive. For 190 patients treated without neoadjuvant trastuzumab, pCR was highest for HR-negative/HER2-positive patients (45%) and lowest for HR-positive/HER2-negative patients (9%). Achieving pCR predicted favorable RFS. For 172 patients treated without trastuzumab, the hazard ratio for RFS of pCR versus no pCR was 0.29 (95% CI, 0.07 to 0.82). pCR was more predictive of RFS by multivariate analysis when subtype was taken into account, and point estimates of hazard ratios within the HR-positive/HER2-negative (hazard ratio, 0.00; 95% CI, 0.00 to 0.93), HR-negative/HER2-negative (hazard ratio, 0.25; 95% CI, 0.04 to 0.97), and HER2-positive (hazard ratio, 0.14; 95% CI, 0.01 to 1.0) subtypes are lower. Ki67 further improved the prediction of pCR within subsets.
Conclusion: In this biologically high-risk group, pCR differs by receptor subset. pCR is more highly predictive of RFS within every established receptor subset than overall, demonstrating that the extent of outcome advantage conferred by pCR is specific to tumor biology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
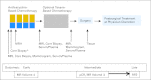
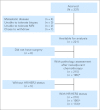

References
-
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. - PubMed
-
- Hser YI. Self-reported drug use: Results of selected empirical investigations of validity. NIDA Res Monogr. 1997;167:320–343. - PubMed
-
- Valero VV, Buzdar AU, Hortobagyi GN. Locally advanced breast cancer. Oncologist. 1996;1:8–17. - PubMed
-
- Esserman LJ, van 't Veer LJ, Perou C, et al. Biology of breast cancers that present as interval cancers and at young age should inform how we approach early detection and prevention. Cancer Res. 2009;69(suppl 2; abstr 6034)
-
- Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- CA58207/CA/NCI NIH HHS/United States
- U01 CA080098/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA079778/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
- U01 CA079778/CA/NCI NIH HHS/United States
- CA079778/CA/NCI NIH HHS/United States
- U10 CA080098/CA/NCI NIH HHS/United States
- CA31964/CA/NCI NIH HHS/United States
- CA080098/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

