Doublecortin (DCX) mediates endocytosis of neurofascin independently of microtubule binding
- PMID: 22649224
- PMCID: PMC3372911
- DOI: 10.1523/JNEUROSCI.5318-11.2012
Doublecortin (DCX) mediates endocytosis of neurofascin independently of microtubule binding
Abstract
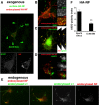
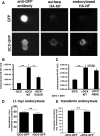
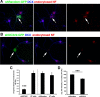
Doublecortin on X chromosome (DCX) is one of two major genetic loci underlying human lissencephaly, a neurodevelopmental disorder with defects in neuronal migration and axon outgrowth. DCX is a microtubule-binding protein, and much work has focused on its microtubule-associated functions. DCX has other reported binding partners, including the cell adhesion molecule neurofascin, but the functional significance of the DCX-neurofascin interaction is not understood. Neurofascin localizes strongly to the axon initial segment in mature neurons, where it plays a role in assembling and maintaining other axon initial segment components. During development, neurofascin likely plays additional roles in axon guidance and in GABAergic synaptogenesis. We show here that DCX can modulate the surface distribution of neurofascin in developing cultured rat neurons and thereby the relative extent of accumulation between the axon initial segment and soma and dendrites. Mechanistically, DCX acts via increasing endocytosis of neurofascin from soma and dendrites. Surprisingly, DCX increases neurofascin endocytosis apparently independently of its microtubule-binding activity. We additionally show that the patient allele DCXG253D still binds microtubules but is deficient in promoting neurofascin endocytosis. We propose that DCX acts as an endocytic adaptor for neurofascin to fine-tune its surface distribution during neuronal development.
Figures



References
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. - PubMed
-
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112:1303–1311. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
