Use of lipid-based nutrient supplements by HIV-infected Malawian women during lactation has no effect on infant growth from 0 to 24 weeks
- PMID: 22649265
- PMCID: PMC3374670
- DOI: 10.3945/jn.111.155598
Use of lipid-based nutrient supplements by HIV-infected Malawian women during lactation has no effect on infant growth from 0 to 24 weeks
Abstract
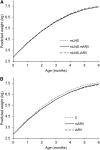
The Breastfeeding, Antiretrovirals, and Nutrition Study evaluated the effect of daily consumption of lipid-based nutrient supplements (LNS) by 2121 lactating, HIV-infected mothers on the growth of their exclusively breast-fed, HIV-uninfected infants from 0 to 24 wk. The study had a 2 × 3 factorial design. Malawian mothers with CD4(+) ≥250 cells/mm(3), hemoglobin ≥70 g/L, and BMI ≥17 kg/m(2) were randomized within 36 h of delivery to receive either no LNS or 140 g/d of LNS to meet lactation energy and protein needs, and mother-infant pairs were assigned to maternal antiretroviral drugs (ARV), infant ARV, or no ARV. Sex-stratified, longitudinal, random effects models were used to estimate the effect of the 6 study arms on infant weight, length, and BMI. Logistic regression models were used to calculate the odds of growth faltering [decline in weight-for-age Z-score (WAZ) or length-for-age Z-score (LAZ) >0.67] using the control arm as the reference. Although some differences between study arms emerged with increasing infant age in boys, there were no consistent effects of the maternal supplement across the 3 growth outcomes in longitudinal models. At the ages where differences were observed, the effects on weight and BMI were quite small (≤200 g and ≤0.4 kg/m(2)) and unlikely to be of clinical importance. Overall, 21 and 34% of infants faltered in WAZ and LAZ, respectively. Maternal supplementation did not reduce the odds of infant weight or length faltering from 0 to 24 wk in any arm. These results indicate that blanket supplementation of HIV-infected lactating women may have little impact on infant growth.
Trial registration: ClinicalTrials.gov NCT00164736.
Conflict of interest statement
Author disclosures: C. M. van der Horst received grant support from Abbott Laboratories and GlaxoSmithKline. V. L. Flax, M. E. Bentley, C. S. Chasela, D. Kayira, M. G. Hudgens, R. J. Knight, A. Soko, D. J. Jamieson, and L. S. Adair, no conflicts of interest.
Figures
Similar articles
-
Antiretroviral Treatment Is Associated With Iron Deficiency in HIV-Infected Malawian Women That Is Mitigated With Supplementation, but Is Not Associated With Infant Iron Deficiency During 24 Weeks of Exclusive Breastfeeding.J Acquir Immune Defic Syndr. 2015 Jul 1;69(3):319-28. doi: 10.1097/QAI.0000000000000588. J Acquir Immune Defic Syndr. 2015. PMID: 25723140 Free PMC article. Clinical Trial.
-
Lipid-based nutrient supplements are feasible as a breastmilk replacement for HIV-exposed infants from 24 to 48 weeks of age.J Nutr. 2013 May;143(5):701-7. doi: 10.3945/jn.112.168245. Epub 2013 Mar 6. J Nutr. 2013. PMID: 23468553 Free PMC article. Clinical Trial.
-
Maternal weight loss during exclusive breastfeeding is associated with reduced weight and length gain in daughters of HIV-infected Malawian women.J Nutr. 2013 Jul;143(7):1168-75. doi: 10.3945/jn.112.171751. Epub 2013 May 22. J Nutr. 2013. PMID: 23700341 Free PMC article. Clinical Trial.
-
Determinants of Child Malnutrition and Infant and Young Child Feeding Approaches in Cambodia.World Rev Nutr Diet. 2016;115:61-7. doi: 10.1159/000444609. Epub 2016 May 19. World Rev Nutr Diet. 2016. PMID: 27197522 Review.
-
Lipid-based nutrient supplements for prevention of child undernutrition: when less may be more.Am J Clin Nutr. 2023 Dec;118(6):1133-1144. doi: 10.1016/j.ajcnut.2023.09.007. Epub 2023 Sep 23. Am J Clin Nutr. 2023. PMID: 37742931
Cited by
-
Plasma and breast-milk selenium in HIV-infected Malawian mothers are positively associated with infant selenium status but are not associated with maternal supplementation: results of the Breastfeeding, Antiretrovirals, and Nutrition study.Am J Clin Nutr. 2014 Apr;99(4):950-6. doi: 10.3945/ajcn.113.073833. Epub 2014 Feb 5. Am J Clin Nutr. 2014. PMID: 24500152 Free PMC article. Clinical Trial.
-
Brief Report: Food Insufficiency Is Associated With Lack of Sustained Viral Suppression Among HIV-Infected Pregnant and Breastfeeding Ugandan Women.J Acquir Immune Defic Syndr. 2016 Mar 1;71(3):310-5. doi: 10.1097/QAI.0000000000000860. J Acquir Immune Defic Syndr. 2016. PMID: 26397935 Free PMC article. Clinical Trial.
-
Antiretroviral Treatment Is Associated With Iron Deficiency in HIV-Infected Malawian Women That Is Mitigated With Supplementation, but Is Not Associated With Infant Iron Deficiency During 24 Weeks of Exclusive Breastfeeding.J Acquir Immune Defic Syndr. 2015 Jul 1;69(3):319-28. doi: 10.1097/QAI.0000000000000588. J Acquir Immune Defic Syndr. 2015. PMID: 25723140 Free PMC article. Clinical Trial.
-
Pre- and post-natal macronutrient supplementation for HIV-positive women in Tanzania: Effects on infant birth weight and HIV transmission.PLoS One. 2018 Oct 11;13(10):e0201038. doi: 10.1371/journal.pone.0201038. eCollection 2018. PLoS One. 2018. PMID: 30307945 Free PMC article. Clinical Trial.
-
Lipid-based nutrient supplements are feasible as a breastmilk replacement for HIV-exposed infants from 24 to 48 weeks of age.J Nutr. 2013 May;143(5):701-7. doi: 10.3945/jn.112.168245. Epub 2013 Mar 6. J Nutr. 2013. PMID: 23468553 Free PMC article. Clinical Trial.
References
-
- UNAIDS. WHO AIDS epidemic update: November 2009. Geneva: UNAIDS; 2009
-
- Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–87 - PubMed
-
- UN The Millenium Development Goals report 2010. New York: United Nations; 2010

