An immunohistochemical method to study breast cancer cell subpopulations and their growth regulation by hormones in three-dimensional cultures
- PMID: 22649363
- PMCID: PMC3355989
- DOI: 10.3389/fendo.2011.00015
An immunohistochemical method to study breast cancer cell subpopulations and their growth regulation by hormones in three-dimensional cultures
Abstract
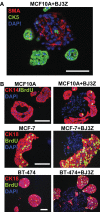
The development of in vitro three-dimensional cell culture matrices offers physiologically relevant alternatives to traditional culture on plastic surfaces. However methods to analyze cell subpopulations therein are poor. Here we present a simple and inexpensive method to analyze cell subpopulations in mixed-cell colonies using standard immunohistochemical (IHC) techniques. Briefly, Matrigel™ blocks are sandwiched between two layers of HistoGel™, hardened by rapid cooling then processed for routine fixation, paraffin embedding, and IHC. We demonstrate the assay using mono- and co-cultured normal human breast, human breast cancer, and transformed mouse stromal cells along with hormone treated breast cancer cells. Judicious selection of specific antibodies allows different cell types within heterotypic colonies to be identified. A brief pulse of bromodeoxyuridine in living colonies allows proliferation of cell subpopulations to be quantified. This simple assay is useful for multiple cell types, species, and conditions.
Keywords: Matrigel; breast cancer; immunohistochemistry; proliferation; three-dimensional culture.
Figures



References
-
- Birgersdotter A., Baumforth K. R., Porwit A., Sundblad A., Falk K. I., Wei W., Sjoberg J., Murray P. G., Bjorkholm M., Ernberg I. (2007). Three-dimensional culturing of the Hodgkin lymphoma cell-line L1236 induces a HL tissue-like gene expression pattern. Leuk. Lymphoma 48, 2042–205310.1080/10428190701573190 - DOI - PubMed
-
- Clemons M., Goss P. (2001). Estrogen and the risk of breast cancer. N. Engl. J. Med. 344, 276–285 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

