Membrane-initiated estradiol signaling regulating sexual receptivity
- PMID: 22649369
- PMCID: PMC3355897
- DOI: 10.3389/fendo.2011.00026
Membrane-initiated estradiol signaling regulating sexual receptivity
Abstract
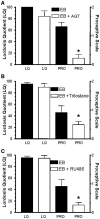
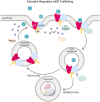
Estradiol has profound actions on the structure and function of the nervous system. In addition to nuclear actions that directly modulate gene expression, the idea that estradiol can rapidly activate cell signaling by binding to membrane estrogen receptors (mERs) has emerged. Even the regulation of sexual receptivity, an action previously thought to be completely regulated by nuclear ERs, has been shown to have a membrane-initiated estradiol signaling (MIES) component. This highlighted the question of the nature of mERs. Several candidates have been proposed, ERα, ERβ, ER-X, GPR30 (G protein coupled estrogen receptor), and a receptor activated by a diphenylacrylamide compound, STX. Although each of these receptors has been shown to be active in specific assays, we present evidence for and against their participation in sexual receptivity by acting in the lordosis-regulating circuit. The initial MIES that activates the circuit is in the arcuate nucleus of the hypothalamus (ARH). Using both activation of μ-opioid receptors (MOR) in the medial preoptic nucleus and lordosis behavior, we document that both ERα and the STX-receptor participate in the required MIES. ERα and the STX-receptor activation of cell signaling are dependent on the transactivation of type 1 metabotropic glutamate receptors (mGluR1a) that augment progesterone synthesis in astrocytes and protein kinase C (PKC) in ARH neurons. While estradiol-induced sexual receptivity does not depend on neuroprogesterone, proceptive behaviors do. Moreover, the ERα and the STX-receptor activation of medial preoptic MORs and augmentation of lordosis were sensitive to mGluR1a blockade. These observations suggest a common mechanism through which mERs are coupled to intracellular signaling cascades, not just in regulating reproduction, but in actions throughout the neuraxis including the cortex, hippocampus, striatum, and dorsal root ganglias.
Keywords: STX; cell signaling; estrogen receptor; lordosis behavior; neuroprogesterone; neurosteroids.
Figures


Similar articles
-
Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats.Endocrinology. 2008 Dec;149(12):5934-42. doi: 10.1210/en.2008-0847. Epub 2008 Jul 24. Endocrinology. 2008. PMID: 18653714 Free PMC article.
-
Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: a role for GABAB receptors.Horm Behav. 2013 Jun;64(1):136-43. doi: 10.1016/j.yhbeh.2013.06.001. Epub 2013 Jun 10. Horm Behav. 2013. PMID: 23756153 Free PMC article.
-
Estradiol dose-dependent regulation of membrane estrogen receptor-α, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats.Endocrinology. 2013 Sep;154(9):3251-60. doi: 10.1210/en.2013-1235. Epub 2013 Jul 3. Endocrinology. 2013. PMID: 23825124 Free PMC article.
-
Membrane-initiated estradiol actions mediate structural plasticity and reproduction.Front Neuroendocrinol. 2012 Oct;33(4):331-41. doi: 10.1016/j.yfrne.2012.07.003. Epub 2012 Jul 22. Front Neuroendocrinol. 2012. PMID: 22828999 Free PMC article. Review.
-
Temporal and concentration-dependent effects of oestradiol on neural pathways mediating sexual receptivity.J Neuroendocrinol. 2013 Nov;25(11):1012-23. doi: 10.1111/jne.12103. J Neuroendocrinol. 2013. PMID: 24028299 Free PMC article. Review.
Cited by
-
Effects and Mechanisms of 3α,5α,-THP on Emotion, Motivation, and Reward Functions Involving Pregnane Xenobiotic Receptor.Front Neurosci. 2012 Jan 19;5:136. doi: 10.3389/fnins.2011.00136. eCollection 2011. Front Neurosci. 2012. PMID: 22294977 Free PMC article.
-
Sexuality and physical contact in National Social Life, Health, and Aging Project Wave 2.J Gerontol B Psychol Sci Soc Sci. 2014 Nov;69 Suppl 2(Suppl 2):S83-98. doi: 10.1093/geronb/gbu072. J Gerontol B Psychol Sci Soc Sci. 2014. PMID: 25360027 Free PMC article.
-
Minireview: neural signaling of estradiol in the hypothalamus.Mol Endocrinol. 2015 May;29(5):645-57. doi: 10.1210/me.2014-1397. Epub 2015 Mar 9. Mol Endocrinol. 2015. PMID: 25751314 Free PMC article. Review. No abstract available.
-
A Novel Membrane Estrogen Receptor Activated by STX Induces Female Sexual Receptivity through an Interaction with mGluR1a.Neuroendocrinology. 2013;97(4):363-8. doi: 10.1159/000351077. Epub 2013 May 22. Neuroendocrinology. 2013. PMID: 23571598 Free PMC article.
-
The Neurosteroid Progesterone Underlies Estrogen Positive Feedback of the LH Surge.Front Endocrinol (Lausanne). 2011 Dec 2;2:90. doi: 10.3389/fendo.2011.00090. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654832 Free PMC article.
References
-
- Beach F. A. (1948). Hormones and Behavior. New York: Paul B. Hoeber, Inc
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

