Drug discovery opportunities and challenges at g protein coupled receptors for long chain free Fatty acids
- PMID: 22649399
- PMCID: PMC3355945
- DOI: 10.3389/fendo.2011.00112
Drug discovery opportunities and challenges at g protein coupled receptors for long chain free Fatty acids
Abstract
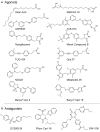
Discovery of G protein coupled receptors for long chain free fatty acids (FFAs), FFA1 (GPR40) and GPR120, has expanded our understanding of these nutrients as signaling molecules. These receptors have emerged as important sensors for FFA levels in the circulation or the gut lumen, based on evidence from in vitro and rodent models, and an increasing number of human studies. Here we consider their promise as therapeutic targets for metabolic disease, including type 2 diabetes and obesity. FFA1 directly mediates acute FFA-induced glucose-stimulated insulin secretion in pancreatic beta-cells, while GPR120 and FFA1 trigger release of incretins from intestinal endocrine cells, and so indirectly enhance insulin secretion and promote satiety. GPR120 signaling in adipocytes and macrophages also results in insulin sensitizing and beneficial anti-inflammatory effects. Drug discovery has focused on agonists to replicate acute benefits of FFA receptor signaling, with promising early results for FFA1 agonists in man. Controversy surrounding chronic effects of FFA1 on beta-cells illustrates that long term benefits of antagonists also need exploring. It has proved challenging to generate highly selective potent ligands for FFA1 or GPR120 subtypes, given that both receptors have hydrophobic orthosteric binding sites, which are not completely defined and have modest ligand affinity. Structure activity relationships are also reliant on functional read outs, in the absence of robust binding assays to provide direct affinity estimates. Nevertheless synthetic ligands have already helped dissect specific contributions of FFA1 and GPR120 signaling from the many possible cellular effects of FFAs. Approaches including use of fluorescent ligand binding assays, and targeting allosteric receptor sites, may improve further pre-clinical ligand development at these receptors, to exploit their unique potential to target multiple facets of diabetes.
Keywords: FFA1; G protein coupled receptor; GPR120; GPR40; adipocytes; free fatty acid; pancreas; type 2 diabetes.
Figures

References
-
- Alquier T., Peyot M. L., Latour M. G., Kebede M., Sorensen C. M., Gesta S., Ronald Kahn C., Smith R. D., Jetton T. L., Metz T. O., Prentki M., Poitout V. (2009). Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 58, 2607–261510.2337/db09-0362 - DOI - PMC - PubMed
-
- Arakawa K., Nishimura T., Sugimoto Y., Takahashi H., Shimamura T. (2010). Novel isoindolin-1-one derivative. International Patent WO 2010/104195.
-
- Bartoschek S., Klabunde T., Defossa E., Dietrich V., Stengelin S., Griesinger C., Carlomagno T., Focken I., Wendt K. U. (2010). Drug design for G-protein-coupled receptors by a ligand-based NMR method. Angew. Chem. Int. Ed. Engl. 49, 1426–1429 - PubMed
-
- Briscoe C. P., Peat A. J., Mckeown S. C., Corbett D. F., Goetz A. S., Littleton T. R., Mccoy D. C., Kenakin T. P., Andrews J. L., Ammala C., Fornwald J. A., Ignar D. M., Jenkinson S. (2006). Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br. J. Pharmacol. 148, 619–62810.1038/sj.bjp.0706770 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

