Insulin analogs and cancer
- PMID: 22649410
- PMCID: PMC3355935
- DOI: 10.3389/fendo.2012.00021
Insulin analogs and cancer
Abstract
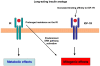
Today, insulin analogs are used in millions of diabetic patients. Insulin analogs have been developed to achieve more physiological insulin replacement in terms of time-course of the effect. Modifications in the amino acid sequence of the insulin molecule change the pharmacokinetics and pharmacodynamics of the analogs in respect to human insulin. However, these changes can also modify the molecular and biological effects of the analogs. The rapid-acting insulin analogs, lispro, aspart, and glulisine, have a rapid onset and shorter duration of action. The long-acting insulin analogs glargine and detemir have a protracted duration of action and a relatively smooth serum concentration profile. Insulin and its analogs may function as growth factors and therefore have a theoretical potential to promote tumor proliferation. A major question is whether analogs have an increased mitogenic activity in respect to insulin. These ligands can promote cell proliferation through many mechanisms like the prolonged stimulation of the insulin receptor, stimulation of the IGF-1 receptor (IGF-1R), prevalent activation of the extracellular-signaling-regulated kinase (ERK) rather than the protein kinase B (PKB/AKT) intracellular post-receptor pathways. Studies on in vitro models indicate that short-acting analogs elicit molecular and biological effects that are similar to those of insulin. In contrast, long-acting analogs behave differently. Although not all data are homogeneous, both glargine and detemir have been found to have a decreased binding to receptors for insulin but an increased binding to IGF-1R, a prevalent activation of the ERK pathway, and an increased mitogenic effect in respect to insulin. Recent retrospective epidemiological clinical studies have suggested that treatment with long-acting analogs (specifically glargine) may increase the relative risk for cancer. Results are controversial and methodologically weak. Therefore prospective clinical studies are needed to evaluate the possible tumor growth-promoting effects of these insulin analogs.
Keywords: IGF-1 receptor; cancer; insulin analogs; insulin receptor; insulin receptor isoforms.
Figures

References
-
- Avnet S., Sciacca L., Salerno M., Gancitano G., Cassarino M. F., Longhi A., Zakikhani M., Carboni J. M., Gottardis M., Giunti A., Pollak M., Vigneri R., Baldini N. (2009). Insulin receptor isoform A and insulin-like growth factor II as additional treatment targets in human osteosarcoma. Cancer Res. 69, 2443–245210.1158/0008-5472.CAN-08-2645 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

