Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo
- PMID: 22649532
- PMCID: PMC3359382
- DOI: 10.1371/journal.pone.0037513
Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo
Abstract
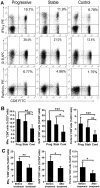
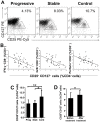
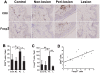
Melanocyte-specific CD8(+) cytotoxic T lymphocytes (CTLs) play a pivotal role in vitiligo-induced depigmentation. Yet, the mechanisms underlying the high frequency of generalized autoimmune disorders associated with generalized vitiligo (GV) are unknown. We hypothesized that an imbalance between activated CD8(+) CTLs and regulatory T cells (Tregs) exists in patients with GV . Assessment of the circulating CD8(+) CTLs and Tregs by flow cytometric analysis revealed an obvious expansion of CD8(+) CTLs and a concomitant decrease in Treg cells in GV patients. The percentages of skin infiltrating CD8(+) CTLs and Tregs were evaluated by immunohistochemistry and revealed dramatically increased numbers of both CD8(+) CTLs and Tregs in the perilesional skin of GV patients. However, peripheral Tregs were impaired in their ability to suppress the proliferation and cytolytic capacity of autologous CD8(+) T cells, suggesting that a functional failure of Tregs and the hyper-activation of CD8(+) CTLs may contribute to progressive GV. Our data indicate that reduced numbers and impaired function of natural Tregs fail to control the widespread activation of CD8(+) CTLs, which leads to the destruction of melanocytes and contributes to the elevated frequency of various associated autoimmune diseases. This knowledge furthers our understanding of the mechanisms of immune tolerance that are impaired in GV patients and may aid in the future development of effective immunotherapy for GV patients.
Conflict of interest statement
Figures

References
-
- Taieb A, Picardo M, Members V. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Research. 2007;20:27–35. - PubMed
-
- Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208–214. - PubMed
-
- Palermo B, Campanelli R, Garbelli S, Mantovani S, Lantelme E, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and Gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. Journal of Investigative Dermatology. 2001;117:326–332. - PubMed
-
- Lang KS, Caroli CC, Muhm A, Wernet D, Moris A, et al. HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. Journal of Investigative Dermatology. 2001;116:891–897. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

