Modeling myocardial infarction in mice: methodology, monitoring, pathomorphology
- PMID: 22649679
- PMCID: PMC3347591
Modeling myocardial infarction in mice: methodology, monitoring, pathomorphology
Abstract
Myocardial infarction is one of the most serious and widespread diseases in the world. In this work, a minimally invasive method for simulating myocardial infarction in mice is described in the Russian Federation for the very first time; the procedure is carried out by ligation of the coronary heart artery or by controlled electrocoagulation. As a part of the methodology, a series of anesthetic, microsurgical and revival protocols are designed, owing to which a decrease in the postoperational mortality from the initial 94.6 to 13.6% is achieved. ECG confirms the development of large-focal or surface myocardial infarction. Postmortal histological examination confirms the presence of necrosis foci in the heart muscles of 87.5% of animals. Altogether, the medical data allow us to conclude that an adequate mouse model for myocardial infarction was generated. A further study is focused on the standardization of the experimental procedure and the use of genetically modified mouse strains, with the purpose of finding the most efficient therapeutic approaches for this disease.
Keywords: ECG (electrocardiogram); controlled electrocoagulation; coronary artery; ligation; myocardial infarction.
Figures


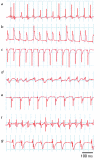
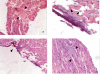
References
-
- Burgueño A.L., Landa M.S., Schuman M.L., Alvarez A.L., Carabelli J., García S.I., Pirola C.J.. Metabolism. 2007;56(10):1439–1443. - PubMed
-
- Cascio W.E., Cozzi E., Hazarika S., Devlin R.B., Henriksen R.A., Lust R.M., van Scott M.R., Wingard C.J.. Inhal Toxicol. 2007;19(5):67–73. - PubMed
-
- Cohen J.K., Cai L.Q., Zhu Y.S., La Perle K.M.. Comp. Med. 2007;57(4):370–376. - PubMed
-
- Denvir M.A., Sharif I., Anderson T., Webb D.J., Gray G.A., McDicken W.N.. J. Am. Soc. Echocardiogr. 2005;18(2):155–162. - PubMed
-
- Lee S., Schwinger R.H., Brixius K.. Pflügers Arch. 2008;455(5):767–774. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
