Role of STAT3 in Transformation and Drug Resistance in CML
- PMID: 22649784
- PMCID: PMC3355894
- DOI: 10.3389/fonc.2012.00030
Role of STAT3 in Transformation and Drug Resistance in CML
Abstract
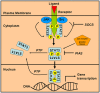
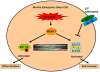
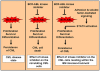
Chronic myeloid leukemia (CML) is initially driven by the bcr-abl fusion oncoprotein. The identification of bcr-abl led to the discovery and rapid translation into the clinic of bcr-abl kinase inhibitors. Although, bcr-abl inhibitors are efficacious, experimental evidence indicates that targeting bcr-abl is not sufficient for elimination of minimal residual disease found within the bone marrow (BM). Experimental evidence indicates that the failure to eliminate the leukemic stem cell contributes to persistent minimal residual disease. Thus curative strategies will likely need to focus on strategies where bcr-abl inhibitors are given in combination with agents that specifically target the leukemic stem cell or the leukemic stem cell niche. One potential target to be exploited is the Janus kinase (JAK)/signal transducers and activators of transcription 3 (STAT3) pathway. Recently using STAT3 conditional knock-out mice it was shown that STAT3 is critical for initiating the disease. Interestingly, in the absence of treatment, STAT3 was not shown to be required for maintenance of the disease, suggesting that STAT3 is required only in the tumor initiating stem cell population (Hoelbl et al., 2010). In the context of the BM microenvironment, STAT3 is activated in a bcr-abl independent manner by the cytokine milieu. Activation of JAK/STAT3 was shown to contribute to cell survival even in the event of complete inhibition of bcr-abl activity within the BM compartment. Taken together, these studies suggest that JAK/STAT3 is an attractive therapeutic target for developing strategies for targeting the JAK-STAT3 pathway in combination with bcr-abl kinase inhibitors and may represent a viable strategy for eliminating or reducing minimal residual disease located in the BM in CML.
Keywords: STAT3; bone marrow microenvironment; chronic myeloid leukemia; drug resistance; transformation.
Figures
References
-
- Aggarwal B. B., Kunnumakkara A. B., Harikumar K. B., Gupta S. R., Tharakan S. T., Koca C., Dey S., Sung B. (2009). Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann. N. Y. Acad. Sci. 1171, 59–76 10.1111/j.1749-6632.2009.04911.x - DOI - PMC - PubMed
-
- Aichberger K. J., Mayerhofer M., Krauth M. T., Skvara H., Florian S., Sonneck K., Akgul C., Derdak S., Pickl W. F., Wacheck V., Selzer E., Monia B. P., Moriggl R., Valent P., Sillaber C. (2005). Identification of Mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and Mcl-1 antisense oligonucleotides. Blood 105, 3303–3311 10.1182/blood-2004-02-0749 - DOI - PubMed
-
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. (1994). Molecular cloning of Aprf, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell 77, 63–71 10.1016/0092-8674(94)90235-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

