Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144
- PMID: 22652344
- PMCID: PMC3530398
- DOI: 10.1016/S1473-3099(12)70088-9
Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144
Abstract
Background: The Thai phase 3 HIV vaccine trial RV 144 showed modest efficacy of a vaccine against HIV acquisition. Baseline variables of age, sex, marital status, and risk did not modify vaccine efficacy. We did a post-hoc analysis of the trial's data to investigate behavioural risk and efficacy every 6 months after vaccination.
Methods: RV 144 was a randomised, multicentre, double-blind, placebo-controlled efficacy trial testing the combination of the HIV vaccines ALVAC-HIV (vCP1521) and AIDSVAX B/E to prevent HIV infection or reduce setpoint viral load. Male and female volunteers aged 18-30 years were recruited from the community. In this post-hoc analysis of the modified intention-to-treat population (16,395 participants), HIV risk behaviour was assessed with a self-administered questionnaire at the time of initial vaccination in the trial and every 6 months thereafter for 3 years. We classified participants' behaviour as low, medium, or high risk. Both the acquisition endpoint and the early viral-load endpoint were examined for interactions with risk status over time and temporal effects after vaccination. Multiple proportional hazards regression models with treatment and time-varying risk covariates were analysed.
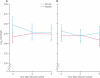
Findings: Risk of acquisition of HIV was low in each risk group, but 9187 (58·2%) participants reported higher-risk behaviour at least once during the study. Participants classified as high or increasing risk at least once during follow-up were compared with those who maintained low-risk or medium-risk behaviour as a time-varying covariate, and the interaction of risk status and acquisition efficacy was significant (p=0·01), with greater benefit in low-risk individuals. Vaccine efficacy seemed to peak early--cumulative vaccine efficacy was estimated to be 60·5% (95% CI 22-80) through the 12 months after initial vaccination--and declined quickly. Vaccination did not seem to affect viral load in either early or late infections.
Interpretation: Future HIV vaccine trials should recognise potential interactions between challenge intensity and risk heterogeneity in both population and treatment effects. The regimen tested in the RV 144 phase 3 trial might benefit from extended immunisation schedules.
Funding: US Army Medical Research and Materiel Command and Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Understanding the efficacy variables of an HIV vaccine trial.Lancet Infect Dis. 2012 Jul;12(7):499-500. doi: 10.1016/S1473-3099(12)70117-2. Epub 2012 May 30. Lancet Infect Dis. 2012. PMID: 22652343 No abstract available.
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. - PubMed
-
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–9. - PubMed
-
- Lee D, Graham BS, Chiu YL, Gilbert PB, McElrath MJ, Belshe RB, et al. Breakthrough infections during phase 1 and 2 prime-boost HIV-1 vaccine trials with canarypox vectors (ALVAC) and booster dose of recombinant gp120 or gp160. J Infect Dis. 2004;190(5):903–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

