Protein-mediated enamel mineralization
- PMID: 22652761
- PMCID: PMC3442115
- DOI: 10.2741/4034
Protein-mediated enamel mineralization
Abstract
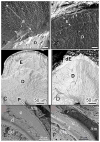
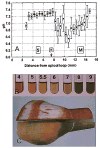
Enamel is a hard nanocomposite bioceramic with significant resilience that protects the mammalian tooth from external physical and chemical damages. The remarkable mechanical properties of enamel are associated with its hierarchical structural organization and its thorough connection with underlying dentin. This dynamic mineralizing system offers scientists a wealth of information that allows the study of basic principels of organic matrix-mediated biomineralization and can potentially be utilized in the fields of material science and engineering for development and design of biomimetic materials. This chapter will provide a brief overview of enamel hierarchical structure and properties and the process and stages of amelogenesis. Particular emphasis is given to current knowledge of extracellular matrix protein and proteinases, and the structural chemistry of the matrix components and their putative functions. The chapter will conclude by discussing the potential of enamel for regrowth.
Figures







References
-
- Lowenstam HA, Weiner S. On Biomineralization. Oxford University Press; New York: 1989.
-
- Moradian-Oldak J, Paine ML. Mammalian Enamel Formation. In: Sigel Astrid, Sigel H, Sigel RKO., editors. Metal Ions In Life Sciences. John Wiley & Sons, Ltd; Chichester: 2008.
-
- Fincham A, Moradian-Oldak J, Simmer J. The structural biology of the developing dental enamel matrix. J Struct Biol. 1999;126(3):270–99. - PubMed
-
- Smith C. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. - PubMed
-
- Robinson C, Kirkham J, Brookes SJ, Bonass WA, Shore RC. The chemistry of enamel development. Int J Dev Biol. 1995;39(1):145–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

