Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures
- PMID: 22653335
- PMCID: PMC3470836
- DOI: 10.1093/eurheartj/ehs095
Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures
Figures
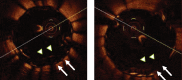
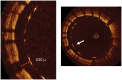
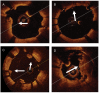
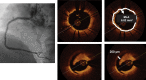
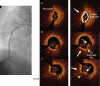
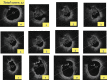
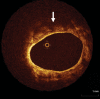
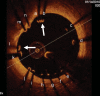
References
-
- Prati F, Regar E, Mintz GS, Arbustini A, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PWJ for the Expert's OCT Review Document. Expert review document on methodology and clinical applications of OCT. Physical principles, methodology of image acquisition and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. - PubMed
-
- Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using Optical Coherence Tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. - PubMed
-
- Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Kitabata H, Kubo T, Nakamura N, Hirata K, Tanaka A, Mizukoshi M, Akasaka T. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75:202–206. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

