C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells
- PMID: 22653339
- PMCID: PMC3469067
- DOI: 10.1038/cdd.2012.67
C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells
Abstract
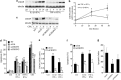
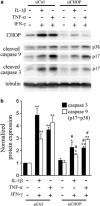
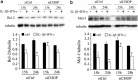
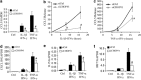
Induction of the C/EBP homologous protein (CHOP) is considered a key event for endoplasmic reticulum (ER) stress-mediated apoptosis. Type 1 diabetes (T1D) is characterized by an autoimmune destruction of the pancreatic β-cells. Pro-inflammatory cytokines are early mediators of β-cell death in T1D. Cytokines induce ER stress and CHOP overexpression in β-cells, but the role for CHOP overexpression in cytokine-induced β-cell apoptosis remains controversial. We presently observed that CHOP knockdown (KD) prevents cytokine-mediated degradation of the anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2) and myeloid cell leukemia sequence 1 (Mcl-1), thereby decreasing the cleavage of executioner caspases 9 and 3, and apoptosis. Nuclear factor-κB (NF-κB) is a crucial transcription factor regulating β-cell apoptosis and inflammation. CHOP KD resulted in reduced cytokine-induced NF-κB activity and expression of key NF-κB target genes involved in apoptosis and inflammation, including iNOS, FAS, IRF-7, IL-15, CCL5 and CXCL10. This was due to decreased IκB degradation and p65 translocation to the nucleus. The present data suggest that CHOP has a dual role in promoting β-cell death: (1) CHOP directly contributes to cytokine-induced β-cell apoptosis by promoting cytokine-induced mitochondrial pathways of apoptosis; and (2) by supporting the NF-κB activation and subsequent cytokine/chemokine expression, CHOP may contribute to apoptosis and the chemo attraction of mononuclear cells to the islets during insulitis.
Figures




References
-
- Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. - PubMed
-
- Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. - PubMed
-
- Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. - PubMed
-
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. - PubMed
-
- Gurzov EN, Eizirik DL. Bcl-2 proteins in diabetes: mitochondrial pathways of beta-cell death and dysfunction. Trends Cell Biol. 2011;21:424–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

