The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization
- PMID: 22653444
- PMCID: PMC3388784
- DOI: 10.1038/embor.2012.73
The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization
Abstract
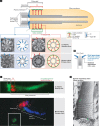
Both the basal body and the microtubule-based axoneme it nucleates have evolutionarily conserved subdomains crucial for cilium biogenesis, function and maintenance. Here, we focus on two conspicuous but underappreciated regions of these structures that make membrane connections. One is the basal body distal end, which includes transition fibres of largely undefined composition that link to the base of the ciliary membrane. Transition fibres seem to serve as docking sites for intraflagellar transport particles, which move proteins within the ciliary compartment and are required for cilium biogenesis and sustained function. The other is the proximal-most region of the axoneme, termed the transition zone, which is characterized by Y-shaped linkers that span from the axoneme to the ciliary necklace on the membrane surface. The transition zone comprises a growing number of ciliopathy proteins that function as modular components of a ciliary gate. This gate, which forms early during ciliogenesis, might function in part by regulating intraflagellar transport. Together with a recently described septin ring diffusion barrier at the ciliary base, the transition fibres and transition zone deserve attention for their varied roles in forming functional ciliary compartments.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Baker K, Beales PL (2009) Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet 151C: 281–295 - PubMed
-
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL (2001) Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol 11: 1586–1590 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

