Quantification of islet size and architecture
- PMID: 22653677
- PMCID: PMC3396703
- DOI: 10.4161/isl.19256
Quantification of islet size and architecture
Abstract
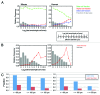
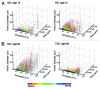
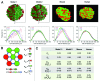
Human islets exhibit distinct islet architecture particularly in large islets that comprise of a relatively abundant fraction of α-cells intermingled with β-cells, whereas mouse islets show largely similar architecture of a β-cell core with α-cells in the periphery. In humans, islet architecture is islet-size dependent. Changes in endocrine cell mass preferentially occurred in large islets as demonstrated in our recent study on pathological changes of the pancreas in patients with type 2 diabetes. ( 1) The size dependency of human islets in morphological changes prompted us to develop a method to capture the representative islet distribution in the whole pancreas section combined with a semi-automated analysis to quantify changes in islet architecture. The computer-assisted quantification allows detailed examination of endocrine cell composition in individual islets and minimizes sampling bias. The standard immunohistochemistry based method is widely applicable to various specimens, which is particularly useful for large animal studies but is also applied to a large-scale analysis of the whole organ section from mice. In this article, we describe the method of image capture, parameters measured, data analysis and interpretation of the data.
Figures

Comment on
- Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, et al. Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PLoS One. 2011;6:e27445. doi: 10.1371/journal.pone.0027445. doi: 10.1371/journal.pone.0027445
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
