Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes
- PMID: 22654089
- PMCID: PMC9487299
- DOI: 10.1183/09059180.00001112
Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes
Abstract
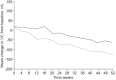
Idiopathic pulmonary fibrosis (IPF) is a progressively fibrotic disease, with no effective treatment and a median survival time of 2-5 yrs. The search for effective treatment has involved numerous clinical trials of investigational agents without significant success until 2011, when European approval was given for the first treatment for IPF, pirfenidone. Four key clinical trials supported the efficacy and tolerability of pirfenidone. In recently published results from two phase III randomised, double-blind, placebo-controlled, multinational trials evaluating pirfenidone (studies 004 and 006), patients with mild-to-moderate IPF were screened for eligibility using the following functional criteria: forced vital capacity (FVC) ≥50% predicted; diffusing capacity of the lung for carbon monoxide ≥35%; and 6-min walk test (6MWT) distance ≥150 m. Only study 004 met the primary end-point of change in per cent predicted FVC at week 72 (p<0.001). Pooled analysis of primary end-point data from both studies also showed that pirfenidone significantly reduced the decline in per cent predicted FVC compared to placebo (p<0.005). Evidence of beneficial effects of pirfenidone treatment was also observed with regard to several secondary end-points, including progression-free survival time, categorical FVC change, and mean change from baseline to week 72 in 6MWT distance. Pirfenidone was generally well tolerated, with the most common side-effects being gastrointestinal discomfort and photosensitivity. The pooled study results, coupled with recent data regarding the prognostic significance of changes in FVC and 6MWT, provide further evidence of a clinically meaningful treatment benefit with pirfenidone in patients with IPF.
Conflict of interest statement
V. Cottin has received fees for speaking from Intermune, Boehringer Ingelheim and Actelion; and has participated as investigator to clinical trials sponsored by InterMune, Boehringer and Actelion, and as a member of a steering committee for a clinical trial sponsored by Boehringer Ingelheim.
Figures

References
-
- Orphanet Report Series. Prevalence of rare disease: bibliographic data. Number 1. 2011. www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_al....
-
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Care Med 2002; 165: 277–304. - PubMed
-
- Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 2010; 35: 496–504. - PubMed
-
- Nathan SD, du Bois RM. Idiopathic pulmonary fibrosis trials: recommendations for the jury. Eur Respir J 2011; 38: 1002–1004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
