Methionine oxidation perturbs the structural core of the prion protein and suggests a generic misfolding pathway
- PMID: 22654104
- PMCID: PMC3436581
- DOI: 10.1074/jbc.M112.354779
Methionine oxidation perturbs the structural core of the prion protein and suggests a generic misfolding pathway
Abstract
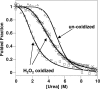
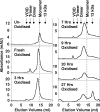
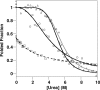
Oxidative stress and misfolding of the prion protein (PrP(C)) are fundamental to prion diseases. We have therefore probed the effect of oxidation on the structure and stability of PrP(C). Urea unfolding studies indicate that H(2)O(2) oxidation reduces the thermodynamic stability of PrP(C) by as much as 9 kJ/mol. (1)H-(15)N NMR studies indicate methionine oxidation perturbs key hydrophobic residues on one face of helix-C as follows: Met-205, Val-209, and Met-212 together with residues Val-160 and Tyr-156. These hydrophobic residues pack together and form the structured core of the protein, stabilizing its ternary structure. Copper-catalyzed oxidation of PrP(C) causes a more significant alteration of the structure, generating a monomeric molten globule species that retains its native helical content. Further copper-catalyzed oxidation promotes extended β-strand structures that lack a cooperative fold. This transition from the helical molten globule to β-conformation has striking similarities to a misfolding intermediate generated at low pH. PrP may therefore share a generic misfolding pathway to amyloid fibers, irrespective of the conditions promoting misfolding. Our observations support the hypothesis that oxidation of PrP destabilizes the native fold of PrP(C), facilitating the transition to PrP(Sc). This study gives a structural and thermodynamic explanation for the high levels of oxidized methionine in scrapie isolates.
Figures
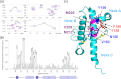






Similar articles
-
Impact of methionine oxidation as an initial event on the pathway of human prion protein conversion.Prion. 2013 Sep-Oct;7(5):404-11. doi: 10.4161/pri.26745. Epub 2013 Oct 9. Prion. 2013. PMID: 24121542 Free PMC article.
-
Roles of methionine oxidation in E200K prion protein misfolding: Implications for the mechanism of pathogenesis in E200K linked familial Creutzfeldt-Jakob disease.Biochim Biophys Acta. 2016 Apr;1864(4):346-58. doi: 10.1016/j.bbapap.2016.01.008. Epub 2016 Jan 15. Biochim Biophys Acta. 2016. PMID: 26779934
-
Oxidation of methionine residues in the prion protein by hydrogen peroxide.Arch Biochem Biophys. 2004 Dec 15;432(2):188-95. doi: 10.1016/j.abb.2004.09.012. Arch Biochem Biophys. 2004. PMID: 15542057
-
Methionine oxidation within the prion protein.Prion. 2020 Dec;14(1):193-205. doi: 10.1080/19336896.2020.1796898. Prion. 2020. PMID: 32744136 Free PMC article. Review.
-
Insights into intragenic and extragenic effectors of prion propagation using chimeric prion proteins.Prion. 2008 Apr-Jun;2(2):45-7. doi: 10.4161/pri.2.2.6509. Epub 2008 Apr 17. Prion. 2008. PMID: 19098443 Free PMC article. Review.
Cited by
-
Methionine Sulfoxide Reductases Suppress the Formation of the [PSI+] Prion and Protein Aggregation in Yeast.Antioxidants (Basel). 2023 Feb 7;12(2):401. doi: 10.3390/antiox12020401. Antioxidants (Basel). 2023. PMID: 36829961 Free PMC article.
-
Conformation-dependent epitopes recognized by prion protein antibodies probed using mutational scanning and deep sequencing.J Mol Biol. 2015 Jan 30;427(2):328-40. doi: 10.1016/j.jmb.2014.10.024. Epub 2014 Nov 7. J Mol Biol. 2015. PMID: 25451031 Free PMC article.
-
Unique methionine-aromatic interactions govern the calmodulin redox sensor.Biochem Biophys Res Commun. 2018 Oct 20;505(1):236-241. doi: 10.1016/j.bbrc.2018.09.052. Epub 2018 Sep 20. Biochem Biophys Res Commun. 2018. PMID: 30243720 Free PMC article.
-
Copper drives prion protein phase separation and modulates aggregation.Sci Adv. 2023 Nov 3;9(44):eadi7347. doi: 10.1126/sciadv.adi7347. Epub 2023 Nov 3. Sci Adv. 2023. PMID: 37922348 Free PMC article.
-
Electron paramagnetic resonance resolves effects of oxidative stress on muscle proteins.Exerc Sport Sci Rev. 2014 Jan;42(1):30-6. doi: 10.1249/JES.0000000000000004. Exerc Sport Sci Rev. 2014. PMID: 24188980 Free PMC article. Review.
References
-
- Milhavet O., Lehmann S. (2002) Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res. Brain Res. Rev. 38, 328–339 - PubMed
-
- Kim J. I., Choi S. I., Kim N. H., Jin J. K., Choi E. K., Carp R. I., Kim Y. S. (2001) Oxidative stress and neurodegeneration in prion diseases. Ann. N.Y. Acad. Sci. 928, 182–186 - PubMed
-
- Martin S. F., Burón I., Espinosa J. C., Castilla J., Villalba J. M., Torres J. M. (2007) Coenzyme Q and protein/lipid oxidation in a BSE-infected transgenic mouse model. Free Radic. Biol. Med. 42, 1723–1729 - PubMed
-
- Yun S. W., Gerlach M., Riederer P., Klein M. A. (2006) Oxidative stress in the brain at early preclinical stages of mouse scrapie. Exp. Neurol. 201, 90–98 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

