A regulatory feedback loop between Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression
- PMID: 22654108
- PMCID: PMC3397910
- DOI: 10.1074/jbc.M112.370783
A regulatory feedback loop between Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression
Abstract
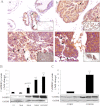
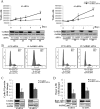
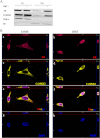
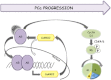
The androgen receptor (AR) plays a critical role in prostate cancer (PCa) progression, however, the molecular mechanisms by which the AR regulates cell proliferation in androgen-dependent and castration-resistant PCa are incompletely understood. We report that Ca(2+)/calmodulin-dependent kinase kinase 2 (CaMKK2) expression increases and becomes nuclear or perinuclear in advanced PCa. In the TRAMP (transgenic adenocarcinoma of mouse prostate) model of PCa, CaMKK2 expression increases with PCa progression with many cells exhibiting nuclear staining. CaMKK2 expression is higher in human castration-resistant tumor xenografts compared with androgen-responsive xenografts and is markedly higher in the AR-expressing, tumorigenic cell line LNCaP compared with cell lines that are AR-nonexpressing and/or nontumorigenic. In LNCaP cells, dihydrotestosterone induced CaMKK2 mRNA and protein expression and translocation of CaMKK2 to the nucleus. Conversely, androgen withdrawal suppressed CaMKK2 expression. Knockdown of CaMKK2 expression by RNAi reduced LNCaP cell proliferation and increased percentages of cells in G(1) phase, whereas correspondingly reducing percentages in S phase, of the cell cycle. CaMKK2 knockdown reduced expression of the AR target gene prostate-specific antigen at both mRNA and protein levels, AR transcriptional activity driven by androgen responsive elements from the prostate-specific probasin gene promoter and levels of the AR-regulated cell cycle proteins, cyclin D1 and hyperphosphorylated Rb. Our results suggest that in PCa progression, CaMKK2 and the AR are in a feedback loop in which CaMKK2 is induced by the AR to maintain AR activity, AR-dependent cell cycle control, and continued cell proliferation.
Figures



References
-
- Colomer J., Means A. R. (2007) Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. Subcell. Biochem. 45, 169–214 - PubMed
-
- Tokumitsu H., Enslen H., Soderling T. R. (1995) Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. J. Biol. Chem. 270, 19320–19324 - PubMed
-
- Edelman A. M., Mitchelhill K. I., Selbert M. A., Anderson K. A., Hook S. S., Stapleton D., Goldstein E. G., Means A. R., Kemp B. E. (1996) Multiple Ca2+/calmodulin-dependent protein kinase kinases from rat brain. J. Biol. Chem. 271, 10806–10910 - PubMed
-
- Kitani T., Okuno S., Fujisawa H. (1997) Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biochem. 122, 243–250 - PubMed
-
- Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. (1998) Components of a calmodulin-dependent protein kinase cascade, molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 273, 31880–31889 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

