Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin β1-mediated expression of phosphorylated focal adhesion kinase
- PMID: 22654119
- PMCID: PMC3406705
- DOI: 10.1074/jbc.M112.349811
Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin β1-mediated expression of phosphorylated focal adhesion kinase
Abstract
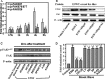
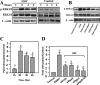
To search for factors promoting bone fracture repair, we investigated the effects of extracorporeal shock wave (ESW) on the adhesion, spreading, and migration of osteoblasts and its specific underlying cellular mechanisms. After a single period of stimulation by 10 kV (500 impulses) of shock wave (SW), the adhesion rate was increased as compared with the vehicle control. The data from both wound healing and transwell tests confirmed an acceleration in the migration of osteoblasts by SW treatment. RT-PCR, flow cytometry, and Western blotting showed that SW rapidly increased the surface expression of α5 and β1 subunit integrins, indicating that integrin β1 acted as an early signal for ESW-induced osteoblast adhesion and migration. It has also been found that a significant elevation occurred in the expression of phosphorylated β-catenin and focal adhesion kinase (FAK) at the site of tyrosine 397 in response to SW stimulation after the increasing expression of the integrin β1 molecule. When siRNAs of integrin α5 and β1 subunit were added, the level of FAK phosphorylation elevated by SW declined. Interestingly, the adhesion and migration of osteoblasts were decreased when these siRNA reagents as well as the ERK1/2 signaling pathway inhibitors, U0126 and PD98059, were present. Further studies demonstrated that U0126 could inhibit the downstream integrin-dependent signaling pathways, such as the FAK signaling pathway, whereas it had no influence on the synthesis of integrin β1 molecule. In conclusion, these data suggest that ESW promotes the adhesion and migration of osteoblasts via integrin β1-mediated expression of phosphorylated FAK at the Tyr-397 site; in addition, ERK1/2 are also important for osteoblast adhesion, spreading, migration, and integrin expression.
Figures






References
-
- Cacchio A., Giordano L., Colafarina O., Rompe J. D., Tavernese E., Ioppolo F., Flamini S., Spacca G., Santilli V. (2009) Extracorporeal shock-wave therapy compared with surgery for hypertrophic long-bone nonunions. J. Bone Joint Surg. Am. 91, 2589–2597 - PubMed
-
- Moretti B., Notarnicola A., Garofalo R., Moretti L., Patella S., Marlinghaus E., Patella V. (2009) Shock waves in the treatment of stress fractures. Ultrasound Med. Biol. 35, 1042–1049 - PubMed
-
- Beutler S., Regel G., Pape H. C., Machtens S., Weinberg A. M., Kremeike I., Jonas U., Tscherne H. (1999) [Extracorporeal shock wave therapy for delayed union of long bone fractures. preliminary results of a prospective cohort study]. Unfallchirurg 102, 839–847 - PubMed
-
- Ikeda K., Tomita K., Takayama K. (1999) Application of extracorporeal shock wave on bone. Preliminary report. J. Trauma 47, 946–950 - PubMed
-
- Wang C. J., Chen H. S., Chen C. E., Yang K. D. (2001) Treatment of nonunions of long bone fractures with shock waves. Clin. Orthop. Relat. Res. 387, 95–101 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

