Distribution of extrasynaptic NMDA receptors on neurons
- PMID: 22654580
- PMCID: PMC3361219
- DOI: 10.1100/2012/267120
Distribution of extrasynaptic NMDA receptors on neurons
Abstract
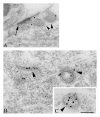
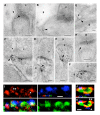
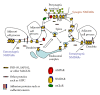
NMDA receptors are found in both synaptic and extrasynaptic locations on neurons. NMDA receptors also can be found on neurons in early stages prior to synaptogenesis, where they may be involved in migration and differentiation. Extrasynaptic NMDA receptors typically are associated with contacts with adjacent processes such as axons and glia. Extrasynaptic NMDA receptor clusters vary in size and may form associations with scaffolding proteins such as PSD-95 and SAP102. The best-characterized extrasynaptic NMDA receptors contain NR1 and NR2B subunits. Extrasynaptic NMDA receptors may be activated by glutamate spillover from synapses or from ectopic release of glutamate. Consequently, extrasynaptic NMDA receptor activation may occur under different circumstances than that for synaptic NMDA receptors, indicating different functional consequences for the neuron. In some cases, activation of extrasynaptic NMDA receptors may have a negative influence on the neuron, leading to cell damage and death, as may occur in some major diseases of the nervous system.
Figures


References
-
- Gereau RW, IV, Swanson GT. The Glutamate Receptors. Totowa, NJ, USA: Humana Press; 2008.
-
- Kehoe J, Buldakova S, Acher F, Dent J, Bregestovski P, Bradley J. Aplysia cys-loop glutamate-gated chloride channels reveal convergent evolution of ligand specificity. Journal of Molecular Evolution. 2009;69(2):125–141. - PubMed
-
- Janovjak H, Sandoz G, Isacoff EY. A modern ionotropic glutamate receptor with a K+ selectivity signature sequence. Nature Communications. 2011;2(1, article 231) - PubMed
-
- Ryan TJ, Grant SGN. The origin and evolution of synapses. Nature Reviews Neuroscience. 2009;10(10):701–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

