The neurotrophins and their role in Alzheimer's disease
- PMID: 22654716
- PMCID: PMC3263452
- DOI: 10.2174/157015911798376190
The neurotrophins and their role in Alzheimer's disease
Abstract
Besides being essential for correct development of the vertebrate nervous system the neurotrophins also play a vital role in adult neuron survival, maintenance and regeneration. In addition they are implicated in the pathogenesis of certain neurodegenerative diseases, and may even provide a therapeutic solution for some. In particular there have been a number of studies on the involvement of nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) in the development of Alzheimer's disease. This disease is of growing concern as longevity increases worldwide, with little treatment available at the moment to alleviate the condition. Memory loss is one of the earliest symptoms associated with Alzheimer's disease. The brain regions first affected by pathology include the hippocampus, and also the entorhinal cortex and basal cholinergic nuclei which project to the hippocampus; importantly, all these areas are required for memory formation. Both NGF and BDNF are affected early in the disease and this is thought to initiate a cascade of events which exacerbates pathology and leads to the symptoms of dementia. This review briefly describes the pathology, symptoms and molecular processes associated with Alzheimer's disease; it discusses the involvement of the neurotrophins, particularly NGF and BDNF, and their receptors, with changes in BDNF considered particularly in the light of its importance in synaptic plasticity. In addition, the possibilities of neurotrophin-based therapeutics are evaluated.
Keywords: Alzheimer's disease; BDNF; NGF; cholinergic basal forebrain.; synaptic plasticity.
Figures


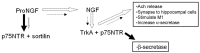
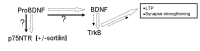
References
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi H M. Forecasting the global burden of Alzheimer's disease. Alzheimers. Dement. 2007;3(3 ):186–191. - PubMed
-
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift fr Psychiatrie Psychisch-Gerichtlich Medizine. 1907;64:146 –148.
-
- Saper C B, German D C, White C L., III Neuronal pathology in the nucleus basalis and associated cell groups in senile dementia of the Alzheimer's type: possible role in cell loss. Neurology. 1985;35(8 ):1089–1095. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4 ):239–259. - PubMed
LinkOut - more resources
Full Text Sources
