Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy
- PMID: 22654805
- PMCID: PMC3356070
- DOI: 10.3389/fendo.2011.00038
Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy
Abstract
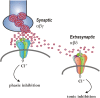
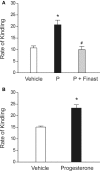
This review highlights the role of major endogenous neurosteroids in seizure disorders and the promise of neurosteroid replacement therapy in epilepsy. Neurosteroids are endogenous modulators of seizure susceptibility. Neurosteroids such as allopregnanolone (3α-hydroxy-5α-pregnane-20-one) and allotetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one) are positive modulators of GABA-A receptors. Aside from peripheral tissues, neurosteroids are synthesized within the brain, mostly in principal neurons. Neurosteroids potentiate synaptic GABA-A receptor function and also activate δ-subunit-containing extrasynaptic GABA-A receptors that mediate tonic currents and thus may play an important role in neuronal network excitability and seizure susceptibility. Our studies over the past decade have shown that neurosteroids are broad-spectrum anticonvulsants and confer seizure protection in various animal models. They protect against seizures induced by GABA-A receptor antagonists, 6-Hz model, pilocarpine-induced limbic seizures, and seizures in kindled animals. Unlike benzodiazepines, tolerance does not occur to their actions during chronic administration. Our recent studies provide compelling evidence that neurosteroids may have antiepileptogenic properties. There is emerging evidence that endogenous neurosteroids may play a key role in the pathophysiology of catamenial epilepsy, stress-sensitive seizure conditions, temporal lobe epilepsy, and alcohol-withdrawal seizures. It is suggested that neurosteroid replacement with natural or synthetic neurosteroids may be useful in the treatment of epilepsy. Synthetic analogs of neurosteroids that are devoid of hormonal side effects show promise in the treatment of diverse seizure disorders. Agents that stimulate endogenous production of neurosteroids may also be useful for treatment of epilepsy.
Keywords: GABA-A receptor; THDOC; allopregnanolone; catamenial epilepsy; epilepsy; epileptogenesis; neurosteroids; seizure.
Figures
Similar articles
-
Neurosteroids — Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy.In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. PMID: 22787590 Free Books & Documents. Review.
-
Neurosteroids and their role in sex-specific epilepsies.Neurobiol Dis. 2014 Dec;72 Pt B:198-209. doi: 10.1016/j.nbd.2014.06.010. Epub 2014 Jun 21. Neurobiol Dis. 2014. PMID: 24960208 Free PMC article. Review.
-
GABA-A Receptors Mediate Tonic Inhibition and Neurosteroid Sensitivity in the Brain.Vitam Horm. 2018;107:177-191. doi: 10.1016/bs.vh.2017.12.001. Epub 2018 Feb 9. Vitam Horm. 2018. PMID: 29544630 Review.
-
Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABAA receptors mediating tonic inhibition and neurosteroid sensitivity.J Neurosci. 2014 Oct 22;34(43):14181-97. doi: 10.1523/JNEUROSCI.0596-14.2014. J Neurosci. 2014. PMID: 25339733 Free PMC article.
-
Neurosteroid replacement therapy for catamenial epilepsy.Neurotherapeutics. 2009 Apr;6(2):392-401. doi: 10.1016/j.nurt.2009.01.006. Neurotherapeutics. 2009. PMID: 19332335 Free PMC article. Review.
Cited by
-
Postnatal administration of allopregnanolone modifies glutamate release but not BDNF content in striatum samples of rats prenatally exposed to ethanol.Biomed Res Int. 2015;2015:734367. doi: 10.1155/2015/734367. Epub 2015 Feb 22. Biomed Res Int. 2015. PMID: 25793205 Free PMC article.
-
Inhibition of human macrophage activation via pregnane neurosteroid interactions with toll-like receptors: Sex differences and structural requirements.Front Immunol. 2022 Jul 29;13:940095. doi: 10.3389/fimmu.2022.940095. eCollection 2022. Front Immunol. 2022. PMID: 35967446 Free PMC article.
-
The dark side of 5α-reductase inhibitors' therapy: sexual dysfunction, high Gleason grade prostate cancer and depression.Korean J Urol. 2014 Jun;55(6):367-79. doi: 10.4111/kju.2014.55.6.367. Epub 2014 Jun 16. Korean J Urol. 2014. PMID: 24955220 Free PMC article. Review.
-
Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders.Life (Basel). 2024 Apr 30;14(5):582. doi: 10.3390/life14050582. Life (Basel). 2024. PMID: 38792602 Free PMC article. Review.
-
Preclinical and clinical pharmacology of brexanolone (allopregnanolone) for postpartum depression: a landmark journey from concept to clinic in neurosteroid replacement therapy.Psychopharmacology (Berl). 2023 Sep;240(9):1841-1863. doi: 10.1007/s00213-023-06427-2. Epub 2023 Aug 11. Psychopharmacology (Berl). 2023. PMID: 37566239 Free PMC article. Review.
References
-
- Bäckström T., Haage D., Löfgren M., Johansson I. M., Strömberg J., Nyberg S., Andréen L., Ossewaarde L., Wingen G. A., Turkmen S., Bengtsson S. K. (2011). Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience 191, 46–5410.1016/j.neuroscience.2011.03.061 - DOI - PubMed
-
- Bauer J., Stoffel-Wagner B., Flügel D., Kluge M., Schramm J., Bidlingmaier F., Elger C. E. (2000). Serum androgens return to normal after temporal lobe epilepsy surgery in men. Neurology 55, 820–824 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

