Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment
- PMID: 22654817
- PMCID: PMC3356006
- DOI: 10.3389/fendo.2011.00060
Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment
Abstract
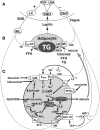
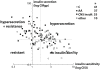
Obesity is a common complication after craniopharyngioma therapy, occurring in up to 75% of survivors. Its weight gain is unlike that of normal obesity, in that it occurs even with caloric restriction, and attempts at lifestyle modification are useless to prevent or treat the obesity. The pathogenesis of this condition involves the inability to transduce afferent hormonal signals of adiposity, in effect mimicking a state of CNS starvation. Efferent sympathetic activity drops, resulting in malaise and reduced energy expenditure, and vagal activity increases, resulting in increased insulin secretion and adipogenesis. Lifestyle intervention is essentially useless in this syndrome, termed "hypothalamic obesity." Pharmacologic treatment is also difficult, consisting of adrenergics to mimic sympathetic activity, or suppression of insulin secretion with octreotide, or both. Recently, bariatric surgery (Roux-en-Y gastric bypass, laparoscopic gastric banding, truncal vagotomy) have also been attempted with variable results. Early and intensive management is required to mitigate the obesity and its negative consequences.
Keywords: craniopharyngioma; ghrelin; hypothalamic obesity; insulin; leptin resistance; octreotide; symapthetic nervous system; vagus nerve.
Figures

References
-
- Arbuzova A., Murray D., McLaughlin S. (1998). MARCKS, membranes, and calmodulin: kinetics of their interaction. Biochim. Biophys. Acta 1376, 369–379 - PubMed
-
- Babinski M. J. (1900). Tumeur du corps pituitaire san acromegalie et avec arret de developpement des organes genitaux. Rev. Neurol. 8, 531–533
-
- Balthasar N., Dalgaard L. T., Lee C. E., Yu J., Funahashi H., Williams T., Ferreira M., Tang V., McGovern R. A., Kenny C. D., Christiansen L. M., Edelstein E., Choi B., Boss O., Aschkenasi C., Zhang C. Y., Mountjoy K., Kishi T., Elmquist J. K., Lowell B. B. (2005). Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–50510.1016/j.cell.2005.08.035 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

