Oxidative Stress-Mediated Brain Dehydroepiandrosterone (DHEA) Formation in Alzheimer's Disease Diagnosis
- PMID: 22654823
- PMCID: PMC3356139
- DOI: 10.3389/fendo.2011.00069
Oxidative Stress-Mediated Brain Dehydroepiandrosterone (DHEA) Formation in Alzheimer's Disease Diagnosis
Abstract
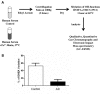
Neurosteroids are steroids made by brain cells independently of peripheral steroidogenic sources. The biosynthesis of most neurosteroids is mediated by proteins and enzymes similar to those identified in the steroidogenic pathway of adrenal and gonadal cells. Dehydroepiandrosterone (DHEA) is a major neurosteroid identified in the brain. Over the years we have reported that, unlike other neurosteroids, DHEA biosynthesis in rat, bovine, and human brain is mediated by an oxidative stress-mediated mechanism, independent of the cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) enzyme activity found in the periphery. This alternative pathway is induced by pro-oxidant agents, such as Fe(2+) and β-amyloid peptide. Neurosteroids are involved in many aspects of brain function, and as such, are involved in various neuropathologies, including Alzheimer's disease (AD). AD is a progressive, yet irreversible neurodegenerative disease for which there are limited means for ante-mortem diagnosis. Using brain tissue specimens from control and AD patients, we provided evidence that DHEA is formed in the AD brain by the oxidative stress-mediated metabolism of an unidentified precursor, thus depleting levels of the precursor in the blood stream. We tested for the presence of this DHEA precursor in human serum using a Fe(2+)-based reaction and determined the amounts of DHEA formed. Fe(2+) treatment of the serum resulted in a dramatic increase in DHEA levels in control patients, whereas only a moderate or no increase was observed in AD patients. The DHEA variation after oxidation correlated with the patients' cognitive and mental status. In this review, we present the cumulative evidence for oxidative stress as a natural regulator of DHEA formation and the use of this concept to develop a blood-based diagnostic tool for neurodegenerative diseases linked to oxidative stress, such as AD.
Keywords: Alzheimer’s disease; dehydroepiandrosterone; diagnostic tool; neurosteroids.
Figures
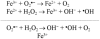
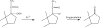

References
-
- Akwa Y., Young J., Kabbadj K., Sancho M. J., Zucman D., Vourc’h C., Jung-Testas I., Hu Z. Y., Le Goascogne C., Jo D. H. (1991). Neurosteroids: biosynthesis, metabolism and function of pregnenolone and dehydroepiandrosterone in the brain. J. Steroid Biochem. Mol. Biol. 40, 71–81 10.1016/0960-0760(91)90169-6 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

