Differentiation of Human Adipose-Derived Stem Cells into "Brite" (Brown-in-White) Adipocytes
- PMID: 22654831
- PMCID: PMC3356055
- DOI: 10.3389/fendo.2011.00087
Differentiation of Human Adipose-Derived Stem Cells into "Brite" (Brown-in-White) Adipocytes
Abstract
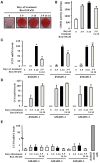
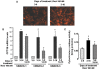
It is well established now that adult humans possess active brown adipose tissue (BAT) which represents a potential pharmacological target to combat obesity and associated diseases. Moreover thermogenic brown-like adipocytes ("brite adipocytes") appear also in mouse white adipose tissue (WAT) upon β3-adrenergic stimulation. We had previously shown that human multipotent adipose-derived stem cells (hMADS) are able to differentiate into cells which exhibit the key properties of human white adipocytes, and then to convert into functional brown adipocytes upon PPARγ activation. In light of a wealth of data indicating that thermogenic adipocytes from BAT and WAT have a distinct cellular origin, we have characterized at the molecular level UCP1 positive hMADS adipocytes from both sexes as brite adipocytes. Conversion of white to brown hMADS adipocytes is dependent on PPARγ activation with rosiglitazone as the most potent agonist and is inhibited by a PPARγ antagonist. In contrast to mouse cellular models, hMADS cells conversion into brown adipocytes is weakly induced by BMP7 treatment and not modulated by activation of the Hedgehog pathway. So far no primary or clonal precursor cells of human brown adipocytes have been obtained that can be used as a tool to develop therapeutic drugs and to gain further insights into the molecular mechanisms of brown adipogenesis in humans. Thus hMADS cells represent a suitable human cell model to delineate the formation and/or the uncoupling capacity of brown/brite adipocytes that could help to dissipate caloric excess intake among individuals.
Keywords: BAT; UCP1; WAT; adipocyte; brite adipocyte; differentiation; rosiglitazone; stem cells.
Figures
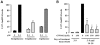
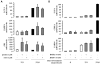
References
-
- Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S. (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E125310.1152/ajpendo.00600.2009 - DOI - PubMed
-
- Bezy O., Elabd C., Cochet O., Petersen R. K., Kristiansen K., Dani C., Ailhaud G., Amri E. Z. (2005). Delta-interacting protein A, a new inhibitory partner of CCAAT/enhancer-binding protein beta, implicated in adipocyte differentiation. J. Biol. Chem. 280, 11432–1143810.1074/jbc.M411741200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

