β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure
- PMID: 22654837
- PMCID: PMC3355892
- DOI: 10.3389/fendo.2011.00102
β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure
Abstract
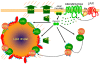
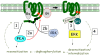
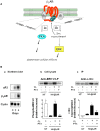
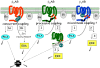
THE ADIPOCYTE IS LIKE A BANK: a place to store excess (caloric) cash in times of plenty, and from which one can withdraw savings during "lean times." The β-adrenoceptors (βAR) are the gateways to this mobilization of fat to be consumed in other tissues. This review discusses the βAR signaling pathway(s) in white and brown adipocytes. Studies in rodent models show that brown adipocytes nestled with white fat depots correlate with and are considered a key enabling factor in resistance to diet-induced obesity. Since it is now recognized that adult humans have brown adipocytes, knowing the steps in these signaling pathways may provide the opportunity to manipulate adipocytes to be net consumers of energy.
Keywords: adipocyte; adrenergic; brown; kinase; signaling; white.
Figures


References
-
- Arch J. R. S., Ainsworth A. T., Ellis R. D., Piercy V., Thody V. E., Thurlby P. L., Wilson C., Wilson S., Young P. (1984). Treatment of obesity with thermogenic β-adrenoceptor agonists: studies on BRL 28630A in rodents. Int. J. Obes. 8, 1–11 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

