Fluorescence In Situ Hybridization for MicroRNA Detection in Archived Oral Cancer Tissues
- PMID: 22654907
- PMCID: PMC3359729
- DOI: 10.1155/2012/903581
Fluorescence In Situ Hybridization for MicroRNA Detection in Archived Oral Cancer Tissues
Abstract
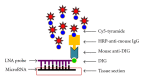
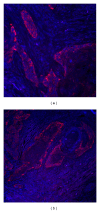
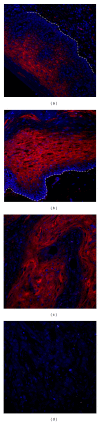
The noncoding RNA designated as microRNA (miRNA) is a large group of small single-stranded regulatory RNA and has generated wide-spread interest in human disease studies. To facilitate delineating the role of microRNAs in cancer pathology, we sought to explore the feasibility of detecting microRNA expression in formalin-fixed paraffin-embedded (FFPE) tissues. Using FFPE materials, we have compared fluorescent in situ hybridization (FISH) procedures to detect miR-146a with (a) different synthetic probes: regular custom DNA oligonucleotides versus locked nucleic acid (LNA) incorporated DNA oligonucleotides; (b) different reporters for the probes: biotin versus digoxigenin (DIG); (c) different visualization: traditional versus tyramide signal amplification (TSA) system; (d) different blocking reagents for endogenous peroxidase. Finally, we performed miR-146a FISH on a commercially available oral cancer tissue microarray, which contains 40 cases of oral squamous cell carcinoma (OSCC) and 10 cases of normal epithelia from the human oral cavity. A sample FISH protocol for detecting miR-146a is provided. In summary, we have established reliable in situ hybridization procedures for detecting the expression of microRNA in FFPE oral cancer tissues. This method is an important tool for studies on the involvement of microRNA in oral cancer pathology and may have potential prognostic or diagnostic value.
Figures

References
-
- Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends in Genetics. 2007;23(5):243–249. - PubMed
-
- Long D, Lee R, Williams P, Chan CY, Ambros V, Ding Y. Potent effect of target structure on microRNA function. Nature Structural and Molecular Biology. 2007;14(4):287–294. - PubMed
-
- Gaur A, Jewell DA, Liang Y, et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Research. 2007;67(6):2456–2468. - PubMed
-
- Zhang L, Yang N, Coukos G. MicroRNA in human cancer: one step forward in diagnosis and treatment. Advances in Experimental Medicine and Biology. 2008;622:69–78. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

