Prenatal hyperandrogenization induces metabolic and endocrine alterations which depend on the levels of testosterone exposure
- PMID: 22655062
- PMCID: PMC3360026
- DOI: 10.1371/journal.pone.0037658
Prenatal hyperandrogenization induces metabolic and endocrine alterations which depend on the levels of testosterone exposure
Abstract
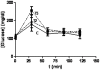
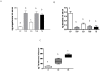
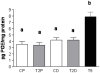
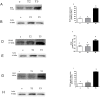
Prenatal hyperandrogenism is able to induce polycystic ovary syndrome (PCOS) in rats. The aim of the present study was to establish if the levels of prenatal testosterone may determine the extent of metabolic and endocrine alterations during the adult life. Pregnant Sprague Dawley rats were prenatally injected with either 2 or 5 mg free testosterone (groups T2 and T5 respectively) from day 16 to day 19 day of gestation. Female offspring from T2 and T5 displayed different phenotype of PCOS during adult life. Offspring from T2 showed hyperandrogenism, ovarian cysts and ovulatory cycles whereas those from T5 displayed hyperandrogenism, ovarian cysts and anovulatory cycles. Both group showed increased circulating glucose levels after the intraperitoneal glucose tolerance test (IPGTT; an evaluation of insulin resistance). IPGTT was higher in T5 rats and directly correlated with body weight at prepubertal age. However, the decrease in the body weight at prepubertal age was compensated during adult life. Although both groups showed enhanced ovarian steroidogenesis, it appears that the molecular mechanisms involved were different. The higher dose of testosterone enhanced the expression of both the protein that regulates cholesterol availability (the steroidogenic acute regulatory protein (StAR)) and the protein expression of the transcriptional factor: peroxisome proliferator-activated receptor gamma (PPAR gamma). Prenatal hyperandrogenization induced an anti-oxidant response that prevented a possible pro-oxidant status. The higher dose of testosterone induced a pro-inflammatory state in ovarian tissue mediated by increased levels of prostaglandin E (PG) and the protein expression of cyclooxygenase 2 (COX2, the limiting enzyme of PGs synthesis). In summary, our data show that the levels of testosterone prenatally injected modulate the uterine environment and that this, in turn, would be responsible for the endocrine and metabolic abnormalities and the phenotype of PCOS during the adult life.
Conflict of interest statement
Figures




References
-
- Franks S. Assessment and management of anovulatory infertility in polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2003;32:639–651. - PubMed
-
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. - PubMed
-
- Franks S. Polycystic ovary syndrome. New England J Med. 1995;333:853–861. - PubMed
-
- Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metabol. 2000;85:2434–2438. - PubMed
-
- Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome- a hypothesis. J Endocrinol. 2002;174:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

