Glutathione s-transferases in pediatric cancer
- PMID: 22655244
- PMCID: PMC3356086
- DOI: 10.3389/fonc.2011.00039
Glutathione s-transferases in pediatric cancer
Abstract
The glutathione S-transferases (GSTs) are a family of ubiquitously expressed polymorphic enzymes important for detoxifying endogenous and exogenous compounds. In addition to their classic activity of detoxification by conjugation of compounds with glutathione, many other functions are now found to be associated with GSTs. The associations between GST polymorphisms/functions and human disease susceptibility or treatment outcome, mostly in adults, have been extensively studied and reviewed. This mini review focuses on studies related to GST epidemiology and functions related to pediatric cancer. Opportunities to exploit GST in pediatric cancer therapy are also discussed.
Keywords: drug resistance; epidemiology; glutathione S-transferase; microsatellite; pediatric cancer; therapeutic target.
Figures
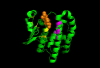

References
-
- Anderer G., Schrappe M., Brechlin A. M., Lauten M., Muti P., Welte K., Stanulla M. (2000). Polymorphisms within glutathione S-transferase genes and initial response to glucocorticoids in childhood acute lymphoblastic leukaemia. Pharmacogenetics 10, 715–72610.1097/00008571-200011000-00006 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

