Quantitative imaging methods for the development and validation of brain biomechanics models
- PMID: 22655600
- PMCID: PMC3711121
- DOI: 10.1146/annurev-bioeng-071811-150032
Quantitative imaging methods for the development and validation of brain biomechanics models
Abstract
Rapid deformation of brain tissue in response to head impact or acceleration can lead to numerous pathological changes, both immediate and delayed. Modeling and simulation hold promise for illuminating the mechanisms of traumatic brain injury (TBI) and for developing preventive devices and strategies. However, mathematical models have predictive value only if they satisfy two conditions. First, they must capture the biomechanics of the brain as both a material and a structure, including the mechanics of brain tissue and its interactions with the skull. Second, they must be validated by direct comparison with experimental data. Emerging imaging technologies and recent imaging studies provide important data for these purposes. This review describes these techniques and data, with an emphasis on magnetic resonance imaging approaches. In combination, these imaging tools promise to extend our understanding of brain biomechanics and improve our ability to study TBI in silico.
Figures




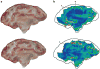

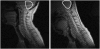

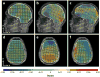
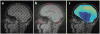


References
-
- Faul M, Xu L, Wald M, Coronado V. Rep, US Dep Health Hum Serv, Cent Dis Control Prev, Natl Cent Inj Prev Control. Atlanta, GA: 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006.
-
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–15. - PubMed
-
- Morrison B, III, Elkin BS, Dollé JP, Yarmush ML. In vitro models of traumatic brain injury. Annu Rev Biomed Eng. 2011;13:91–126. - PubMed
-
- Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10:47–54. - PubMed
-
- Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53:799–812. discussion 812–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

