Anisotropic fibrous scaffolds for articular cartilage regeneration
- PMID: 22655795
- PMCID: PMC3463280
- DOI: 10.1089/ten.TEA.2011.0606
Anisotropic fibrous scaffolds for articular cartilage regeneration
Abstract
Articular cartilage lesions, which can progress to osteoarthritis, are a particular challenge for regenerative medicine strategies, as cartilage function stems from its complex depth-dependent microstructural organization, mechanical properties, and biochemical composition. Fibrous scaffolds offer a template for cartilage extracellular matrix production; however, the success of homogeneous scaffolds is limited by their inability to mimic the cartilage's zone-specific organization and properties. We fabricated trilaminar scaffolds by sequential electrospinning and varying fiber size and orientation in a continuous construct, to create scaffolds that mimicked the structural organization and mechanical properties of cartilage's collagen fibrillar network. Trilaminar composite scaffolds were then compared to homogeneous aligned or randomly oriented fiber scaffolds to assess in vitro cartilage formation. Bovine chondrocytes proliferated and produced a type II collagen and a sulfated glycosaminoglycan-rich extracellular matrix on all scaffolds. Furthermore, all scaffolds promoted significant upregulation of aggrecan and type II collagen gene expression while downregulating that of type I collagen. Compressive testing at physiological strain levels further demonstrated that the mechanical properties of trilaminar composite scaffolds approached those of native cartilage. Our results demonstrate that trilaminar composite scaffolds mimic key organizational characteristics of native cartilage, support in vitro cartilage formation, and have superior mechanical properties to homogenous scaffolds. We propose that these scaffolds offer promise in regenerative medicine strategies to repair articular cartilage lesions.
Figures


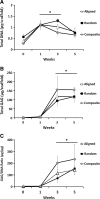


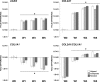


References
-
- Daher R.J. Chahine N.O. Greenburg A.S. Sgagilone N.A. Grande D.A. New methods to diagnose and treat cartilage degeneration. Nat Rev Rheumatology. 2009;5:599. - PubMed
-
- Becerra J. Andrades J.A. Guerado E. Zamora-Navas P. Lopez-Puertas J.M. Reddi H. Articular cartilage: structure and regeneration. Tissue Eng Part B. 2010;16:617. - PubMed
-
- Erggelet C. Kreuz P.C. Mrosek E.H. Schagemann J.C. Lahm A. Ducommun P.P., et al. Autologous chondrocyte implantation versus ACI using 3D-bioresorbably graft for the treatment of large full-thickness cartilage lesions of the knee. Arch Orthop Trauma Surg. 2010;130:957. - PubMed
-
- Nuernberger S. Cyran N. Albrecht C. Redl H. Vecsei V. Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32:1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

