Iron transport-mediated drug delivery: practical syntheses and in vitro antibacterial studies of tris-catecholate siderophore-aminopenicillin conjugates reveals selectively potent antipseudomonal activity
- PMID: 22656303
- PMCID: PMC3380153
- DOI: 10.1021/ja303446w
Iron transport-mediated drug delivery: practical syntheses and in vitro antibacterial studies of tris-catecholate siderophore-aminopenicillin conjugates reveals selectively potent antipseudomonal activity
Abstract
An artificial tris-catecolate siderophore with a tripodal backbone and its conjugates with ampicillin and amoxicillin were synthesized. Both conjugates exhibited significantly enhanced in vitro antibacterial activities against Gram-negative species compared to the parent drugs, especially against Pseudomonas aeruginosa . The conjugates appeared to be assimilated by an induced bacterial iron transport process as their activities were inversely related to iron concentration. The easily synthesized tris-catecolate siderophore has great potential for future development of various drug conjugates to target antibiotic-resistant Gram-negative bacteria.
Figures
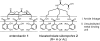
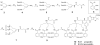
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

